High performance liquid chromatography analysis method for measuring sodium sulfite content
A high-performance liquid chromatography and sodium sulfite technology, applied in the field of drug detection, can solve problems such as inseparability, poor solution stability, and low sensitivity, and achieve high recovery, high sensitivity, and short analysis time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The selection of embodiment 1 chromatographic conditions
[0031] 1.1 Selection of wavelength
[0032] Take the standard solution, use the mobile phase as the blank calibration solution, and scan at 190nm to 400nm according to the ultraviolet-visible spectrophotometric method (Appendix IV A of Part Two of the Chinese Pharmacopoeia 2010 Edition). The result shows that need testing solution has maximum absorption peak at 214nm place. Therefore, 214nm±5nm was determined as the detection wavelength.
[0033] 1.2 Method screening test
[0034] In this test, the chromatographic method of this test is screened by adjusting the composition and ratio of the mobile phase, pH value, flow rate, column temperature, etc. See Table 1 for specific plans.
[0035] Table 1 Screening scheme of the method
[0036]
[0037] Precisely measure 1ml of sodium thiosulfate injection, put it in a 25ml measuring bottle, add mobile phase to dilute to the mark, shake well, and use it as the...
Embodiment 2
[0042] The stability of embodiment 2 solution
[0043] Take by weighing about 14 mg of anhydrous sodium sulfite, accurately weigh it, put it in a 25ml measuring bottle, dissolve it with the mobile phase in Scheme 3 in Example 1 and dilute to the mark, shake it up, and use it as reference solution 1; in addition, take anhydrous sodium sulfite About 14mg, accurately weighed, put in 25ml measuring bottle, dissolve and dilute to scale with the mobile phase in scheme 6 in embodiment 1, shake up, as reference substance solution two.
[0044] Precisely measure 5 μl of the reference substance solution at 0 hour, 2 hours, 4 hours, 6 hours, and 8 hours, test according to the chromatographic conditions of scheme 3, and record the chromatograms. In addition, at 0 hour, 2 hours, 4 hours, 6 hours, and 8 hours, 5 μl of the reference substance solution was accurately measured, and the test was carried out according to the chromatographic conditions of scheme 6, and the chromatograms were re...
Embodiment 3
[0049] Embodiment 3 The making of standard curve
[0050] Chromatographic conditions: the same as the chromatographic conditions described in Scheme 3 in Example 1.
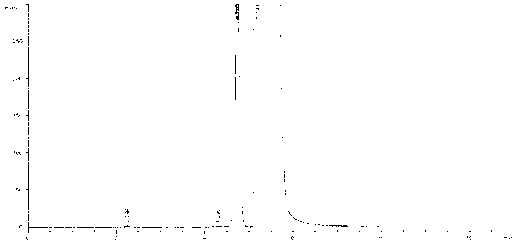
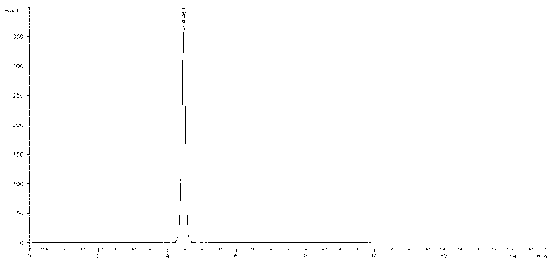
[0051]Weigh 28.1mg, 21.7mg, 14.3mg, and 7.6mg of anhydrous sodium sulfite, put them in 25ml measuring bottles, add an appropriate amount of mobile phase, shake to dissolve, add mobile phase to the scale, shake well, and use it as a linear solution 1 to 4 . Precisely measure 1ml each of linear solution 1 and linear solution 3, respectively, put them in 10ml measuring bottles, add mobile phase to the mark, shake well, and use them as linear solutions 5 and 6. For the chromatogram of linear solution 3 see figure 2 .
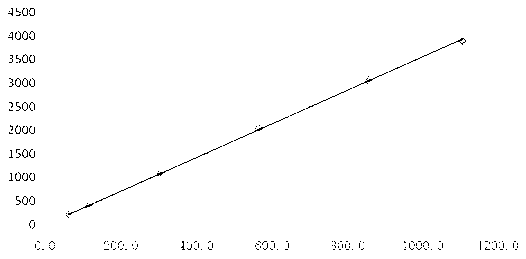
[0052] Take the linear solution of sodium sulfite for injection, and plot the peak area and concentration (see image 3 ). Sodium sulfite solution is linear in the range of 56.0 μg / ml to 1100.4 μg / ml, and the linear regression equation is:
[0053] A=3.5518C+22.0731, correlation coefficient r=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com