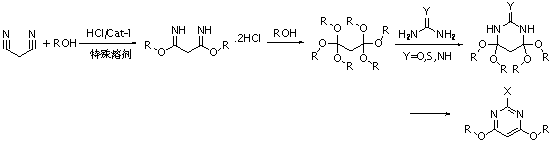
Novel synthesis process of 2-substituted-4,6-dialkoxy pyrimidine
A dialkoxypyrimidine and new process technology, which is applied in the field of new synthesis process of 2-substituted-4,6-dialkoxypyrimidine, which can solve the problems of difficult industrial production, harsh operating conditions, easy decomposition and polymerization, etc. problem, to achieve the effect of promoting alcoholysis rate, low production cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Synthesis of dimethoxymalonamidine hydrochloride
[0042] At room temperature, add malononitrile (132.0g / 2.0mol) and methyl acetate solution (1000ml) of methanol (320.0g / 10.0mol) and solid titanium tetrachloride (2.6g) in a 2L four-necked flask , stir and mix well, pass dry hydrogen chloride gas into the system, keep the pressure of 0.1-0.5MPa, keep the temperature at 25-35°C for reaction and stir for 4 hours, stop the reaction, degas, filter, and dry the filter cake to obtain 436.2g of white solid. Content 91.3% (GC-Area% after derivatization), single-step yield 98.1%, structure by 1 Confirmed by H-NMR.
[0043] (2) Synthesis of hexamethoxypropane
[0044] At room temperature, add dimethoxypropanediamidine hydrochloride (218.1g / 0.98mol) and 1300ml methanol (dried with sodium metal) into a 2L four-neck flask, stir and mix well, and control the pH of the mixture to be 3.5~4, keep warm at 40~45°C for 24 hours, cool down to room temperature, filter and precipitate, ...
Embodiment 2
[0049] (1) Synthesis of dimethoxymalonamidine hydrochloride
[0050] Add malononitrile (132.0g / 2.0mol) and methanol (320.0g / 10.0mol) in methyl acetate solution (1000ml) and zirconium oxide (3.0g) in a 2L four-necked flask, cool to 0~ Stir and mix well at 10°C, pass dry hydrogen chloride gas into the system, keep the pressure of 0.1-0.5MPa, stir for 7 hours, stop the reaction, degas, filter, and dry the filter cake to obtain 417.5g of white solid with a content of 91.9% (derivative GC-Area% after chemical reaction), the single-step yield is 94.5%, and the structure 1 Confirmed by H-NMR.
[0051] (2) Synthesis of hexamethoxypropane
[0052] At room temperature, add dimethoxypropanediamidine hydrochloride (220.9g / 1.0mol) and 1300ml methanol (dried with sodium metal) into a 2L four-neck flask, stir and mix well, and control the pH of the mixture to be 2-3, keep warm at 40-45°C for 31 hours, cool down to room temperature, filter and remove the solvent, add 550ml ethyl acetate to...
Embodiment 3
[0057] (1) Synthesis of dimethoxymalonamidine hydrochloride
[0058] At room temperature, add malononitrile (132.0g / 2.0mol) and methyl tert-butyl ether solution (1000ml) of methanol (320.0g / 10.0mol) and cobalt chloride (1.5g) in a 2L four-necked flask, Stir and mix well, pass dry hydrogen chloride gas into the system, keep the pressure of 0.1~0.5MPa, keep the temperature at 25~35°C for reaction and stir for 5 hours, stop the reaction, degas, filter, and dry the filter cake to obtain 421.0g of white solid, content 92.2% (GC-Area% after derivatization), single-step yield 95.6%, structure 1 Confirmed by H-NMR.
[0059] (2) Synthesis of hexamethoxypropane
[0060] Add dimethoxypropanediamidine hydrochloride (210.5g / 0.96mol) and 1300ml methanol (dried with sodium metal) into a 2L four-necked flask, stir and mix, and control the pH value of the mixture to 3.5-4. Heat the reaction at 20-25°C for 40 hours, filter and remove the solvent, add 550ml of ethyl acetate to the concentrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com