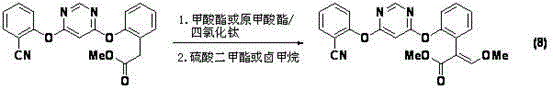
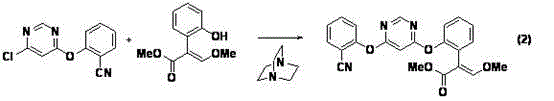Azoxystrobin synthesis method
A synthetic method, azoxystrobin technology, applied in the field of chemical product production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
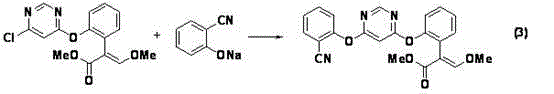
[0038] Preparation of Example 1 4-chloro-6-(2-cyanophenoxy)pyrimidine:
[0039](1) 13.8g (0.10mol) of 2-chlorobenzonitrile, 10.65g (0.095mol) of 4,6-dihydroxypyrimidine, 13.8g (0.10mol) of anhydrous potassium carbonate, 0.5gCuCl (0.005mol) and 50mL Sulfolane was added into a 250ml four-neck flask reactor, and the reaction was carried out under nitrogen protection. Raise the temperature to 95°C for 1 hour, then raise the temperature to 135°C for 13 hours. Then it was slowly lowered to room temperature, separated by filtration, and the solvent was removed to obtain 19.64 g of a light yellow solid, which was 4-hydroxy-6-(2-cyanophenoxy)pyrimidine.
[0040] (ii) Add 63.4ml (0.45mol) of phosphorus oxychloride to the above 19.64g of solid 4-hydroxy-6-(2-cyanophenoxy)pyrimidine, cool down to 0°C, and dropwise add 40.4g (0.2mol) of triethylamine After dripping, keep warm for 2h, slowly raise the temperature to 80°C, keep warm and reflux for 5h. The reaction mixture was cooled and t...
example 2
[0042] Example 2 Preparation of 4-chloro-6-(2-cyanophenoxy)pyrimidine
[0043] (1) 13.8g (0.10mol) 2-chlorobenzonitrile, 12.78g (0.114mol) 4,6-dihydroxypyrimidine, 14.49g (0.105mol) anhydrous potassium carbonate, 0.5g (0.005mol) CuCl and 50mLN- Methylpyrrolidone was added into a 250ml four-necked flask reactor, and reacted under nitrogen protection. Raise the temperature to 95°C for 1 hour, then raise the temperature to 140°C for 11 hours. Then it was slowly lowered to room temperature, separated by filtration, and the solvent was removed to obtain 20.4 g of a light yellow solid, which was 4-hydroxy-6-(2-cyanophenoxy)pyrimidine.
[0044] (ii) Add 63.4ml (0.45mol) of phosphorus oxychloride to the above 20.4g of solid 4-hydroxy-6-(2-cyanophenoxy)pyrimidine, cool down to 0°C, and dropwise add 40.4g (0.2mol) of triethylamine After dripping, keep warm for 2h, slowly raise the temperature to 80°C, keep warm and reflux for 5h. The reaction mixture was cooled and the solvent was re...
example 3
[0046] Example 3 Preparation of (E)-2-{2-[6-(2-cyanophenoxy)pyrimidin-4-yloxy]phenyl}-3-methoxymethyl acrylate (compound I):
[0047] In a 250ml four-necked flask reactor, 21.0g (0.10mol) (E)-2-(2-hydroxyphenyl)-3-methoxymethyl acrylate and 25.48g (0.11mol) 4-chloro-6- (2-cyanophenoxy)pyrimidine was dissolved in 60mL N,N-dimethylformamide, 20.7g (0.15mol) of anhydrous potassium carbonate and 0.36g (0.003mol) of 4-dimethylaminopyridine were added, in Under the protection of nitrogen, the temperature was raised to 60° C. for 3.5 h. After cooling down to room temperature, a brown-red solid was obtained through precipitation. The solid was dissolved in 100mL of toluene, added with 50mL of water, washed and concentrated under reduced pressure (using a water bath temperature of 60°C) to obtain 38.2g of a crude product with a content of 92.0% and a yield of 87.2%.
[0048] Add 40ml of methanol to 38.2g of the above crude product, heat up to 70°C and reflux for 2h, cool down to 0-5°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com