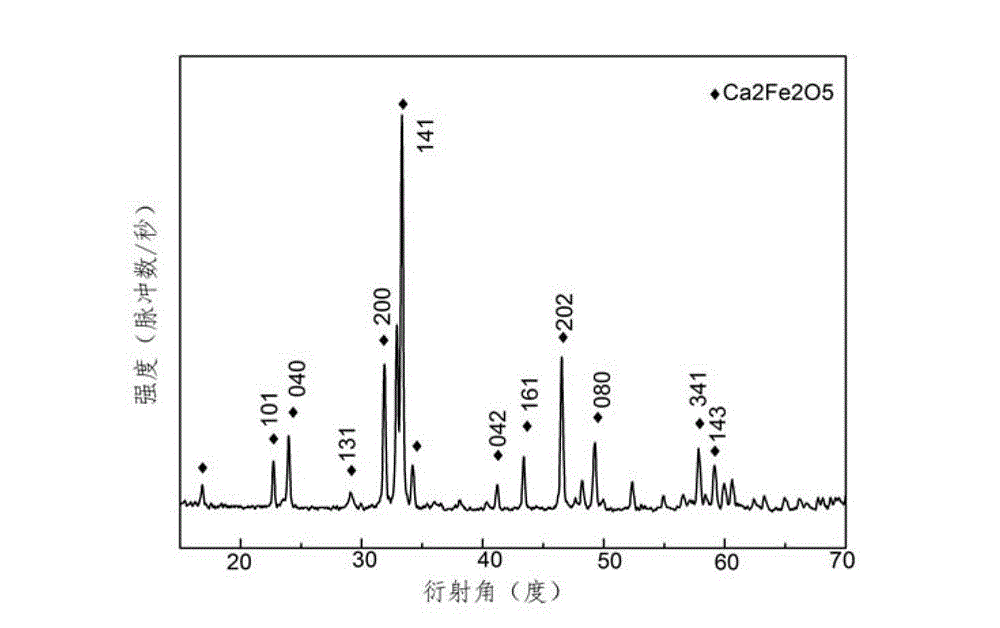
Method for preparing Ca2Fe2O5 nano powder
A technology of ca2fe2o5 and nanopowder, which is applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of difficult control of the process from sol to gel, long calcination temperature and holding time, unfavorable energy saving and environmental protection, etc. Achieve the effects of cheap raw materials, lower calcination temperature and good crystallization performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Prepare ferric nitrate and calcium nitrate with a concentration of 0.5mol / L and calcium nitrate with a concentration of 0.5mol / L in a 250ml volumetric flask, respectively;
[0024] 2) Pipette 20mL of ferric nitrate solution and 20mL of calcium nitrate solution and mix evenly to obtain mixed solution A; wherein, the molar ratio of iron ions to calcium ions in mixed solution A is 1:1;
[0025] 3) According to the propulsion chemistry theory, add glycine as a combustion accelerant to mixed solution A, and stir evenly with a magnetic stirrer for 10 minutes to obtain mixed solution B; wherein, the amount of glycine added is the ferric nitrate and 1.5 times the total molar weight of calcium nitrate;
[0026] 4) Put the mixed solution B into the muffle furnace and heat it to 150°C to obtain a dark brown fluffy primary powder, then grind the dark brown fluffy primary powder evenly, and then heat it to 700°C in the muffle furnace Heat preservation and roasting for 0.5h to ob...
Embodiment 2
[0028] 1) Prepare ferric nitrate and calcium nitrate with a concentration of 0.1mol / L and calcium nitrate with a concentration of 0.1mol / L in a 250ml volumetric flask, respectively;
[0029] 2) Pipette 20mL of ferric nitrate solution and 20mL of calcium nitrate solution and mix evenly to obtain mixed solution A; wherein, the molar ratio of iron ions to calcium ions in mixed solution A is 1:1;
[0030] 3) According to the propulsion chemistry theory, add glycine as a combustion accelerant to mixed solution A, and stir evenly with a magnetic stirrer for 10 minutes to obtain mixed solution B; wherein, the amount of glycine added is the ferric nitrate and 2.8 times the total molar weight of calcium nitrate;
[0031] 4) Put the mixed solution B into the muffle furnace and heat it to 200°C to obtain a dark brown fluffy primary powder, then grind the dark brown fluffy primary powder evenly, and then heat it to 600°C in the muffle furnace Heat preservation and roasting for 1.0h to ob...
Embodiment 3
[0036] 1) Prepare ferric nitrate and calcium nitrate with a concentration of 0.2 mol / L and 0.2 mol / L calcium nitrate in a 250ml volumetric flask, respectively;
[0037] 2) Pipette 20mL of ferric nitrate solution and 20mL of calcium nitrate solution and mix evenly to obtain mixed solution A; wherein, the molar ratio of iron ions to calcium ions in mixed solution A is 1:1;
[0038] 3) According to the propulsion chemistry theory, add glycine as a combustion accelerant to mixed solution A, and stir evenly with a magnetic stirrer for 10 minutes to obtain mixed solution B; wherein, the amount of glycine added is the ferric nitrate and 2.0 times the total molar weight of calcium nitrate;
[0039] 4) Heat the mixed solution B to 200°C in a muffle furnace and keep it warm for 1 hour to obtain a dark brown fluffy primary powder, then grind the dark brown fluffy primary powder evenly, and then heat it in the muffle furnace Roast at 500°C for 1.0h to obtain dark brown Ca 2 Fe 2 o 5 N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com