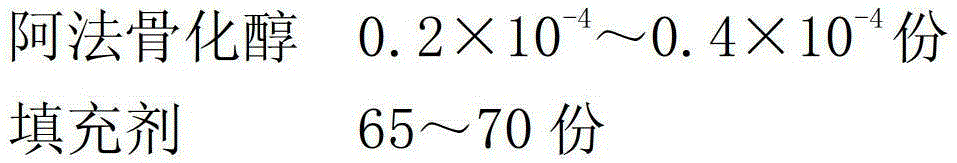Alfacalcidol sustained-release granule and preparation method thereof
A technology of alfacalcidol and sustained-release granules, which is applied in bone diseases, medical formulas, medical preparations of non-active ingredients, etc., and can solve the problems of absorption, unsatisfactory treatment effect, short administration time interval, and blood drug concentration. Large fluctuations and other problems, to achieve the effect of stable product quality, reduced dosage, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
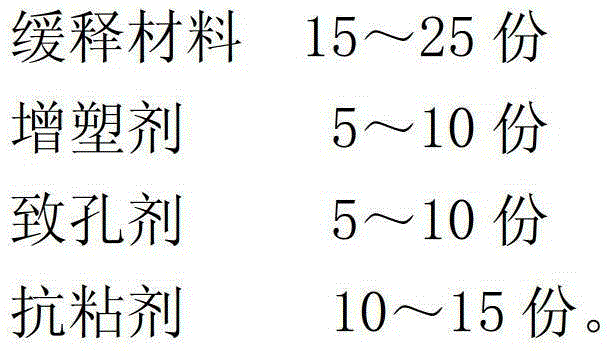
[0021] A preparation method for alfacalcidol sustained-release granules, comprising the following steps:
[0022] (1) Mix alfacalcidol and filler evenly, and use 85% ethanol as a binder to make immediate-release granule cores with 10-14 meshes for later use;
[0023] (2) Dissolving the slow-release material, plasticizer, porogen, and anti-tack agent with 80% ethanol to make a slow-release coating solution;
[0024] (3) Evenly spray the formulated sustained-release coating solution on the surface of the immediate-release granule core prepared in step (1), and dry to obtain alfacalcidol sustained-release granules.
Embodiment 1~4
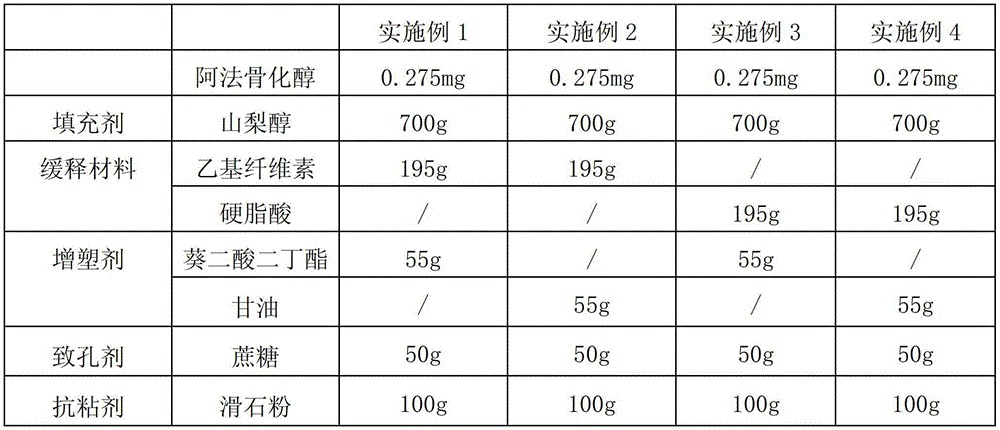
[0025] Preparation of Examples 1-4 Alfacalcidol Sustained Release Granules
[0026] According to the raw and auxiliary materials in the following table, the alfacalcidol sustained-release granules of four examples were prepared according to the above-mentioned preparation method. Among them, " / " means not used.
[0027]
[0028] Test Example 1 Determination of Release of Alfacalcidol Sustained-release Granules Gained in Examples 1-4
[0029] According to the "Guidelines for Sustained and Controlled Release Preparations" in the appendix of the 2010 edition of the Pharmacopoeia of the People's Republic of China (Part II), 0.25% sodium lauryl sulfate was used as the release medium, and the preparations in Examples 1 to 4 were accurately weighed. The appropriate amount of alfacalcidol sustained-release granules (about 100 mg) was determined according to the first method of appendix XD of "Pharmacopoeia of the People's Republic of China" 2010 edition, and the peak area was dete...
Embodiment 5~7
[0034] Embodiments 5-7 Preparation of alfacalcidol sustained-release granules
[0035] According to the raw and auxiliary materials in the following table, according to the above-mentioned preparation method, alfacalcidol sustained-release granules were prepared in each embodiment. The weight ratio of the slow-release material of embodiment 5 and the plasticizer is 4:1, the weight ratio of the slow-release material of embodiment 6 and the plasticizer is 3:1, and the weight ratio of the slow-release material of embodiment 7 and the plasticizer The weight ratio is 2:1.
[0036]
[0037] Test Example 2 Determination of release rate of alfacalcidol sustained-release granules obtained in Examples 5-7
[0038] The measuring method is the same as that of Test Example 1. The measurement results are shown in Table 2.
[0039] Table 2 Examination of the Release Rate of Alfacalcidol Sustained-release Granules in Examples 5-7 (Dissolution Medium: 0.25% Sodium Lauryl Sulfate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com