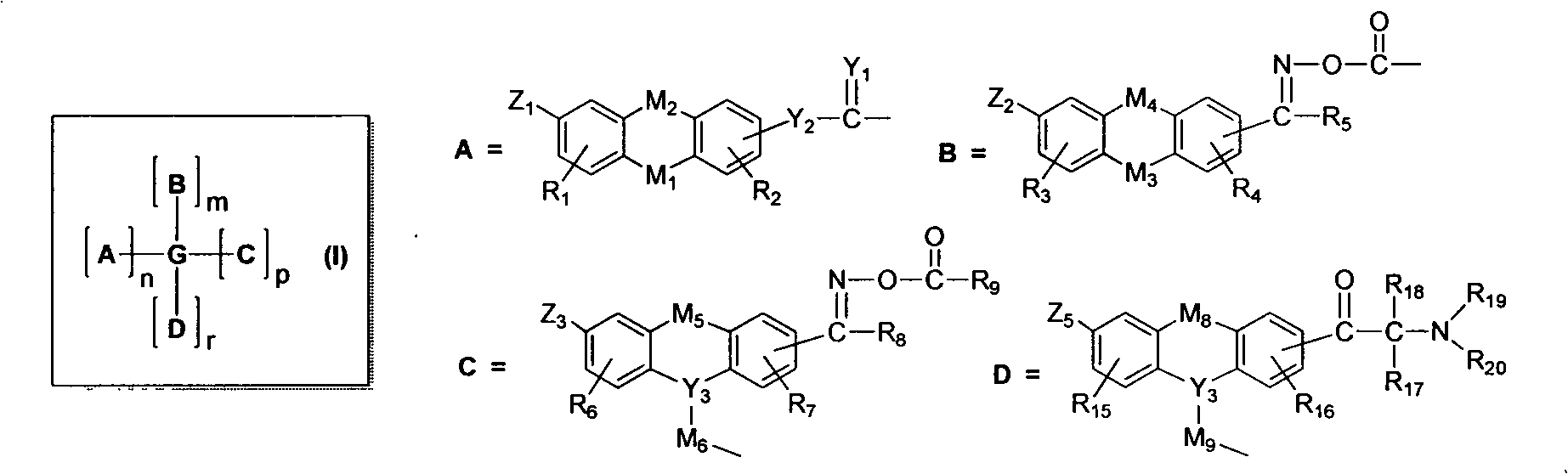
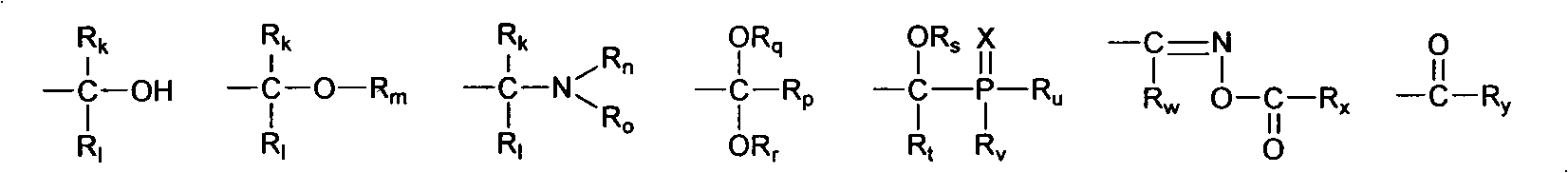
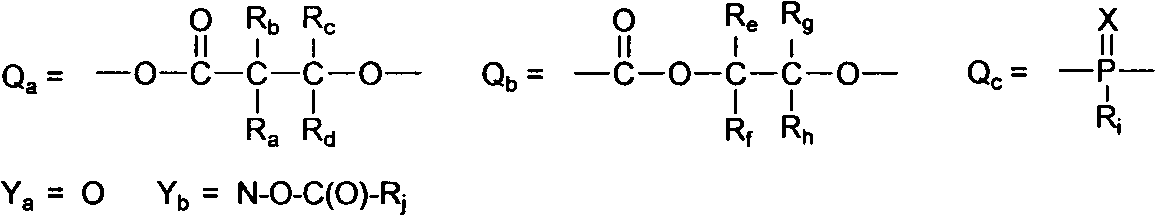Soluble oxime ester and aromatic ketone photo polymerization initiator
A technology of definition and substituent, applied in the field of soluble oxime ester and aromatic ketone photopolymerization initiators, can solve the problems of practical application limitation, insufficient solubility, difficult to dissolve and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089]
[0090] Step 1: Place 2.4 g of NaOH in 50 ml of dry DMF under an ice-water bath, stir for 10 minutes, then add 5 g of carbazole in batches and continue stirring for half an hour, then add 2.7 ml of bromoethane dropwise. After the dropwise addition, the ice-water bath was removed and stirring was continued at room temperature for 5-8 hours (TLC tracking). After the reaction was completed, the reaction solution was slowly poured into an ice-water bath, extracted 4 times with 100 ml of ethyl acetate each time, the extract was collected, dried over sodium sulfate, and concentrated by rotary evaporation to obtain a crude product. Dissolve it in an appropriate amount of dichloromethane (until it dissolves), spread 5 cm thick silica gel in the Buchner funnel, filter, and wash the silica gel layer 3 times with dichloromethane (20 ml each time) (TLC detection) , the mother liquor was collected, concentrated by rotary evaporation and dried to obtain 5.6 g of N-ethylcarbazole ...
Embodiment 2
[0115]
[0116] With reference to the reaction conditions in the first to fifth steps of Example 1, only benzoyl chloride was used to replace the acetyl chloride in Example 1 in the final esterification step of the fifth step, and the light yellow target product was obtained with a yield of 92%.
[0117] Target product NMR data:
[0118] 1 H-NMR (CDCl 3 , 400MHz): 8.90(s, 1H), 8.48(s, 1H), 8.37(dd, 1H, J=9.2Hz, J=2Hz), 7.92(dd, 1H), 7.77(m, 3H), 7.78( m, 3H), 7.69-7.26 (m, 16H), 4.39 (q, 2H, J=7.2Hz), 1.47ppm (t, 3H, J=7.2Hz);
[0119] 13 C-NMR (CDCl 3 , 100MHz): 163.9, 163.8, 143.4, 142.3, 141.1, 136.1, 133.7, 133.6, 132.7, 132.2, 132.1, 132.0, 131.9, 131.8, 131.7, 131.6, 131.5, 130.5, 130.5, 129.9, 129.9 7 ppm.
Embodiment 3
[0121]
[0122] The first step: the same as the first step in embodiment one;
[0123] The second step: similar to the second step of Example 1, the only difference is that the acylation reagent is changed to CH 3 C(O)C(O)Cl, the product was a pale yellow solid.
[0124] NMR data:
[0125] 1 H-NMR (CDCl 3 , 400MHz): 8.82(s, 1H), 8.20-8.17(m, 2H), 7.58-7.55(dd, 1H), 7.49-7.46(m, 2H), 7.35(dd, 1H), 4.42(q, 2H , J=7.2Hz), 2.62(s, 3H), 1.49ppm(t, 3H, J=7.2Hz);
[0126] 13 C-NMR (CDCl 3 , 100MHz): 202.0, 191.4, 143.6, 140.7, 128.0, 126.8, 126.5, 124.5, 123.2, 123.1, 122.8, 120.9, 120.5, 109.2, 108.7, 37.9, 26.8, 13.8ppm.
[0127] The third to fifth steps: the same as the third to fifth steps of Example 1; 2.7 g of the light yellow target compound was prepared with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com