Flunarizine derivative, preparation method and use thereof
A technology of flunarizine and derivatives, which is applied in the field of pharmacy and can solve problems such as clinical use restrictions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
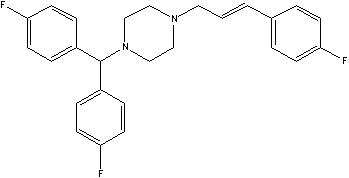
[0064] Embodiment one: the preparation of triflunarizine
[0065] Dissolve 9g of p-fluorocinnamyl chloride in 150ml of ethanol, 18g of di-p-fluorobenzylpiperazine in 150ml of ethanol, add them together to the reactor, heat to reflux, keep reflux for 2 hours, evaporate the ethanol to dryness, and dissolve the residue in ethyl acetate Esters—extracted with water, washed the organic phase with water, washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain triflunarizine C as a light yellow solid 26 h 25 f 3 N 2 . Elemental analysis results: C: 73.80%; H: 5.95%; F: 13.51%; N: 6.67%. Calculated values: C: 73.92%; H: 5.96%; F: 13.49%; N: 6.63%. Proton NMR spectrum: 7.01-7.30 (6H); 7.53-7.62 (6H); 6.75 (1H); 6.44 (1H); 4.50 (1H); 3.92 (2H); 2.53-2.8098H).
[0066] The corresponding salt can be obtained by further reaction of triflunarizine with acid.
Embodiment 2
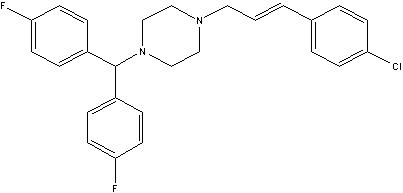
[0067] Embodiment two: replace the p-fluorocinnamyl chloride in embodiment one with p-chlorocinnamyl chloride, use similar method, can obtain chloroflunarizine C 26 h 25 CIF 2 N 2. Elemental analysis results: C: 71.20%; H: 5.68%; Cl: 8.12%; F: 8.65%; N: 6.35%. Calculated: C: 71.15%; H: 5.73%; Cl: 8.08%; F: 8.66%; N: 6.68%. Proton NMR spectrum: 7.21-7.52 (12H); 6.71 (1H); 6.45 (1H); 4.5 (1H); 3.42 (2H); 2.5-2.8 (8H). Chlorflunarizine can be further reacted with acid to obtain the corresponding salt.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com