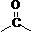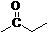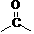Aryl carboxylic acid imatinib derivative as well as preparation and application thereof
A technology of aryl carboxylic acid and imatinib, which is applied in the field of derivatives with anti-tumor activity and their preparation, can solve the problems of no anti-tumor application, poor anti-chronic myeloid leukemia activity, and the like, and achieves structural Novel, obvious inhibitory effect, mild synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 (E)-3-(4-trifluoromethylphenyl)-N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidine-2-amino))benzamide
[0026]
[0027] Weigh 4-trifluoromethylbenzoic acid (2.67 g, 15 mmol) and dissolve it in 30 mL of dichloromethane, add oxalyl chloride (2.20 g, 18 mmol) dropwise under ice-cooling, stir at room temperature for 3 h, and 2- [N-(2-Methyl-5-aminophenyl)amino]-4-(3-pyridyl)pyrimidine (3.78 g, 13.5 mmol) was dissolved in 30 mL of dichloromethane and added to it. After 8 hours of reaction, the reaction was complete , filtered, collected the solid matter, and washed three times with dichloromethane to obtain 5.61 g of off-white solid with a yield of 84.2% and a melting point of 218-220 °C. The NMR data of the product are as follows: 1 H NMR (300 MHz, DMSO-d 6 )δ: 10.49 (s, 1H); 9.28 (s, 1H); 9.00 (s, 1H); 8.69-8.68 (t, 1H, J=4.4Hz); 8.54-8.45 (m, 2H); 8.16-8.12 (t, 3H, J=16.4Hz); 7.92-7.90 (d, 2H, J=8.4Hz); 7.55-7.50(m, 2H); 7.45-7.43(d, 1H, J=5.2Hz); 7.24- 7.22(d, 1...
Embodiment 2
[0028] Example 2 N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidine-2-amino))-(4-methylphenyl)formamide
[0029]
[0030] The operation steps are the same as in Example 1, except that 4-methylbenzoic acid is used instead of 4-trifluoromethylbenzoic acid. The target product is a light yellow solid with a yield of 84.7% and a melting point of 211-213 ℃. The NMR data of the product are as follows: 1 H NMR (300 MHz, DMSO-d 6 )δ: 10.14 (s, 1H); 9.27 (s, 1H); 9.07-8.94 (m, 1H); 8.73 (s, 1H); 8.54-8.49 (m, 2H); 8.10-7.01 (m, 2H) ;7.88-7.85(t, 1H, J=12.0Hz); 8.53-7.32(m, 5H); 7.23-7.20(d, 1H, J=10.4Hz); 2.38(s, 3H); 2.23(s, 3H ).
Embodiment 3
[0031] Example 3 N-(4-methyl-3-(4-(pyridin-3-yl)pyrimidine-2-amino))-(4-nitrophenyl)formamide
[0032]
[0033] The operation steps are the same as in Example 1, except that 4-nitrobenzoic acid is used instead of 4-trifluoromethylbenzoic acid. The target product is a brown solid with a yield of 82.5% and a melting point of 253-258°C. The NMR data of the product are as follows: 1 H NMR (400 MHz, DMSO-d 6 )δ: 10.63 (s, 1H); 9.45 (s, 1H); 9.22 (s, 1H); 9.08-9.01 (m, 3H); 8.72-8.70 (d, 2H, J=8.4Hz); 8.11-7.01 (m, 3H); 7.62-7.50 (m, 3H); 7.26-7.25 (d, 1H, J=6.4Hz); 2.26 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com