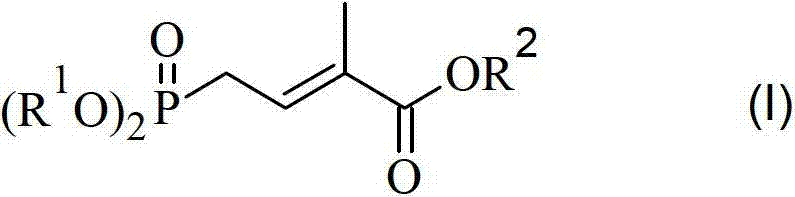
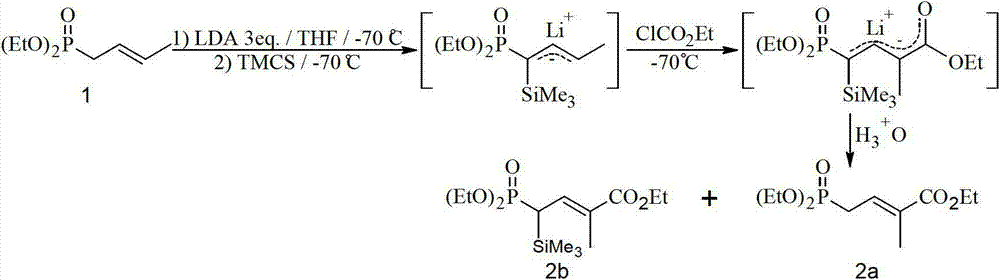
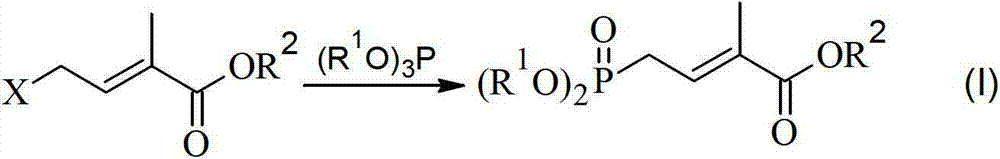Method for preparing 4-bialkoxy-phosphono-2-methyl-2-butenoic acid alkyl ester
A technology of dihydrocarbyloxyphosphono and crotonate hydrocarbyl esters, which is applied in the fields of chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve problems such as suboptimal methods, heavy pollution, Process complexity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 41.0g (0.22mol) diethyl 2-oxoethylphosphate and 80.0g (0.22mol) ethyl 2-triphenylphosphine ylide propionate in 400ml dichloromethane under nitrogen protection and 25°C , stirred and heated to 40° C. for reflux reaction for 20 hours. After the reaction was completed, dichloromethane was removed by concentration under reduced pressure, 200ml of n-heptane was added and the temperature was lowered to 0°C to precipitate triphenylphosphine oxide crystals, which were removed by filtration. The filtrate was concentrated under reduced pressure to remove the solvent to obtain 67.9 g of a yellow oil, mainly 4-diethoxyphosphono-2-methyl-2-butenoic acid ethyl ester, which was purified by a silica gel column to obtain 51.0 g of the product, Yield 88%.
Embodiment 2
[0037]Under nitrogen protection and 25°C, dissolve 41.0g (0.22mol) of 2-oxoethyl diethyl phosphate in 100ml of dichloromethane, then add 80.0g (0.22mol) of 2-triphenylphosphine dropwise under stirring A solution of ethyl ylide propionate dissolved in 400 ml of dichloromethane took about 120 minutes. After the dropwise addition was completed, the temperature was raised to 40° C. for reflux reaction for 10 hours. After the reaction was completed, dichloromethane was removed by concentration under reduced pressure, 200ml of n-heptane was added and the temperature was lowered to 0°C to precipitate triphenylphosphine oxide crystals, which were removed by filtration. The filtrate was concentrated under reduced pressure to obtain 66.2g of yellow oil, mainly 4-diethoxyphosphono-2-methyl-2-butenoic acid ethyl ester, purified by silica gel column to obtain 48.8g of product, the yield 84%.
Embodiment 3
[0039] Dissolve 34.5g (0.22mol) dimethyl 2-oxoethylphosphate and 80.0g (0.22mol) ethyl 2-triphenylphosphine ylide propionate in 400ml dichloromethane under nitrogen protection and 25°C , reflux reaction for 20 hours. After the reaction was completed, dichloromethane was removed by concentration under reduced pressure, 200ml of n-heptane was added and the temperature was lowered to 0°C to precipitate triphenylphosphine oxide crystals, which were removed by filtration. The filtrate was concentrated under reduced pressure to remove the solvent to obtain 64.2 g of a yellow oil, mainly 4-dimethoxyphosphono-2-methyl-2-butenoic acid ethyl ester, which was purified by a silica gel column to obtain 44.6 g of the product, Yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com