Pharmaceutical composition of injection ceftizoxime sodium and compound amino acid injection
A technology of ceftizoxime sodium and compound amino acids, which is applied in the field of medicine and can solve problems such as slow intravenous injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
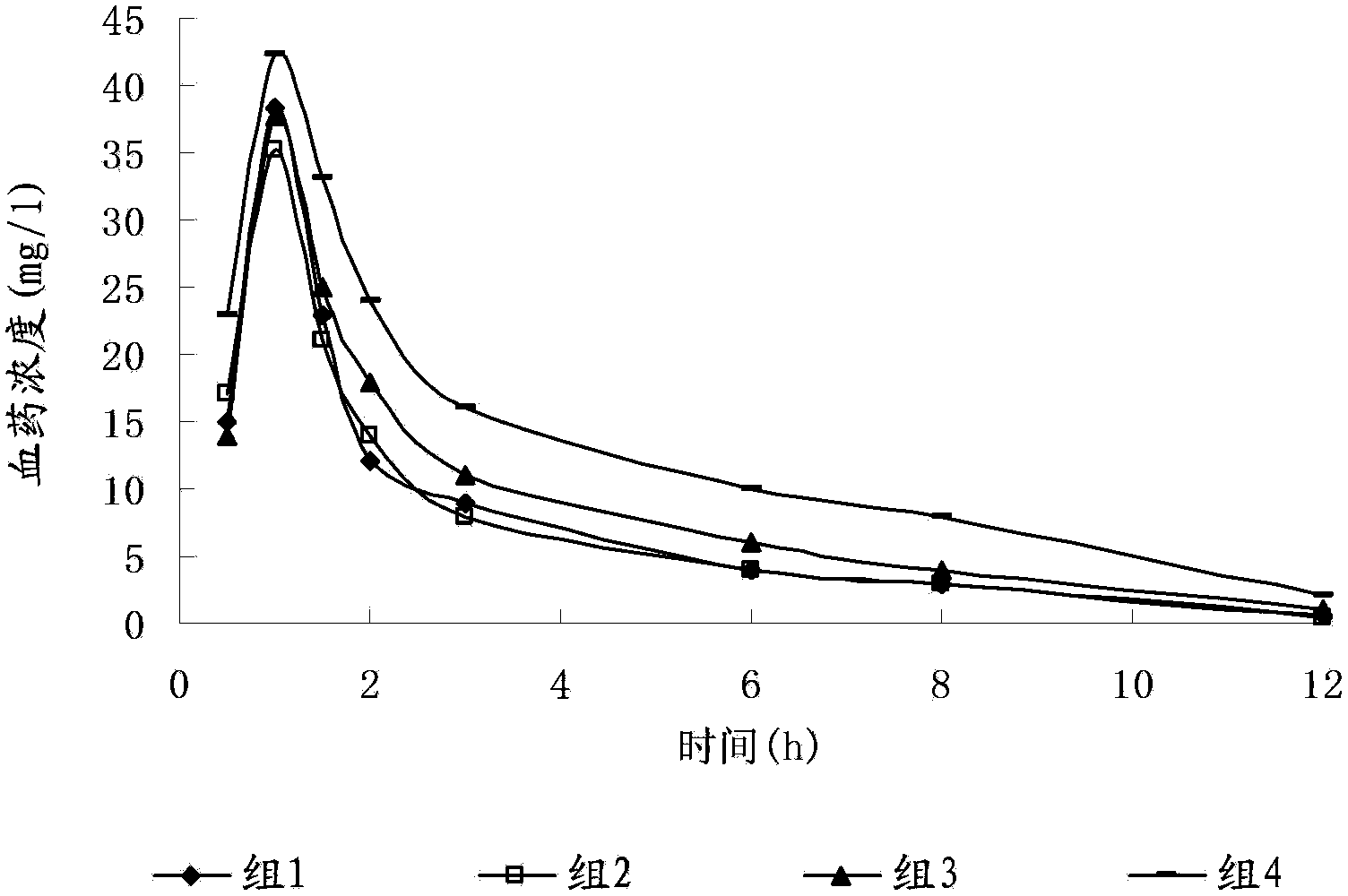
Image
Examples
Embodiment 1
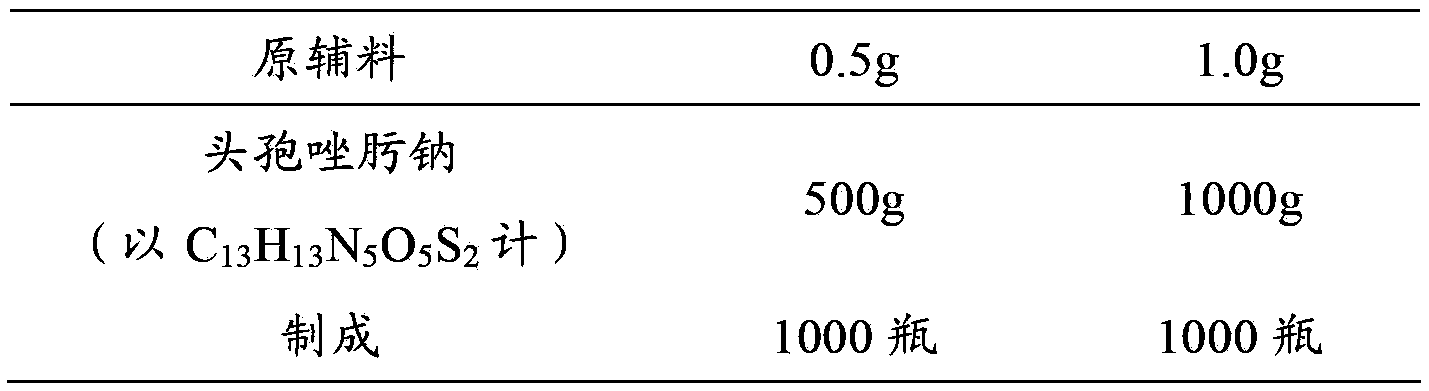
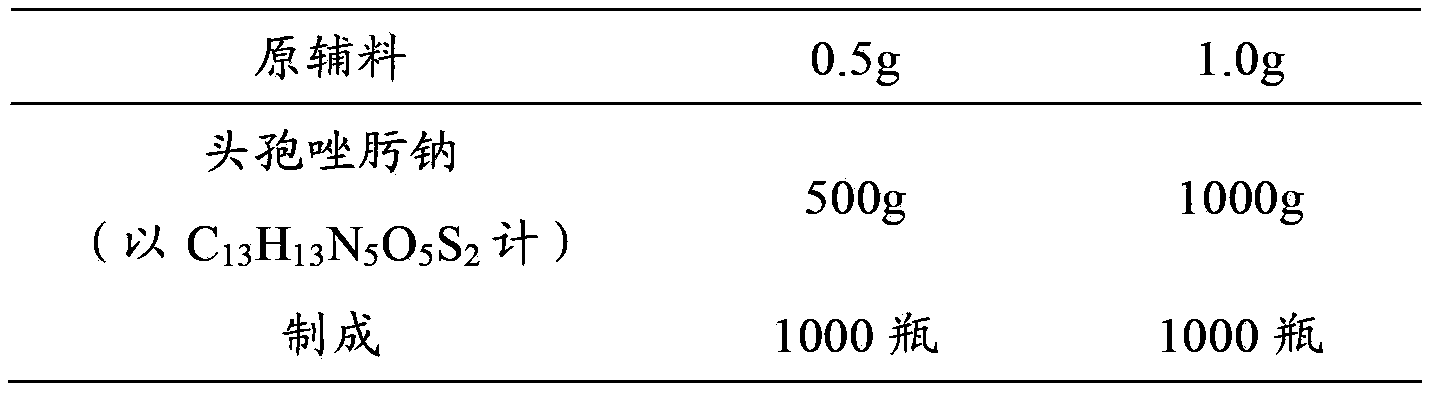
[0031] Example 1 Preparation of Ceftizoxime Sodium for Injection
[0032] prescription:
[0033]
[0034] Preparation Process:
[0035] (1) Ceftizoxime sodium sterile powder 500g (in C 13 h 13 N 5 o 5 S 2 meter) into the subpackaging room (the ambient temperature of the subpackaging post is 18°C-26°C, and the relative humidity is below 65%), debug the subpackaging machine, adjust the number of steps to adjust the loading volume, so that the loading volume reaches the specified range, and then distribute Put it in a low borosilicate glass control injection bottle, press the stopper completely, and crimp the cap.
[0036] (2) Ceftizoxime sodium sterile powder 1000g (in C 13 h 13 N 5 o 5 S 2 meter) into the subpackaging room (the ambient temperature of the subpackaging post is 18°C-26°C, and the relative humidity is below 65%), debug the subpackaging machine, adjust the number of steps to adjust the loading volume, so that the loading volume reaches the specified ...
Embodiment 2
[0037] Example 2 Preparation of Compound Amino Acid Injection
[0038] prescription:
[0039]
[0040](1) Take 60L of water for injection and boil it, add 5000g of xylitol to dissolve it, then add 150g of activated carbon and boil for 20 minutes, filter back to decolorize for 20 minutes, then filter into dilute irrigation.
[0041] (2) After filtering the xylitol liquid into the dilution tank, nitrogen gas is introduced immediately, and at the same time, another 40 L of water for injection that has been pretreated with 150 g of activated carbon for 20 minutes of boiling and adsorption is combined with the xylitol liquid, and the cooling water is used When the combined solution was cooled to 60°C, feeding began.
[0042] (3) Divide 50g of the antioxidant sodium bisulfite into two equal parts, put 25g of one part of the antioxidant into the combined liquid, then add 289g of arginine hydrochloride and 246g of histidine hydrochloride according to the order of the above formu...
Embodiment 3
[0045] Example 3 Preparation of Combination Packaged Drugs
[0046] Combination 1: Ceftizoxime Sodium for Injection 0.5g and Compound Amino Acid Injection 100ml.
[0047] Combination 2: Ceftizoxime Sodium for Injection 0.5g and Compound Amino Acid Injection 250ml.
[0048] Combination 3: Ceftizoxime Sodium for Injection 1.0g and Compound Amino Acid Injection 250ml.
[0049] Combination 4: Ceftizoxime Sodium for Injection 1.0g and Compound Amino Acid Injection 500ml.
[0050] Combination 5: Ceftizoxime Sodium for Injection 2.0g and Compound Amino Acid Injection 250ml.
[0051] Combination 6: Ceftizoxime Sodium for Injection 2.0g and Compound Amino Acid Injection 500ml.
[0052] Combination 7: 1.0 g of ceftizoxime sodium for injection and 100 ml of compound amino acid injection.
[0053] Combination 8: Ceftizoxime Sodium for Injection 3.0g and Compound Amino Acid Injection 250ml.
[0054] Combination 9: Ceftizoxime Sodium for Injection 3.0g and Compound Amino Acid Injecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com