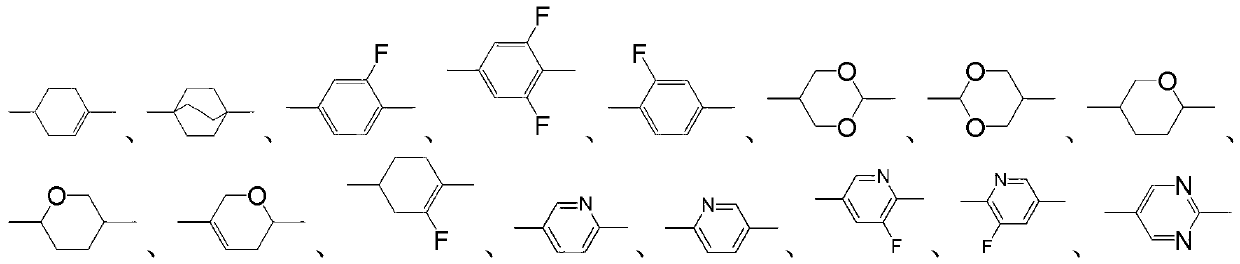
Benzofuran derivative liquid crystal compound as well as composition and application thereof
A liquid crystal compound, an independent technology, applied in liquid crystal materials, chemical instruments and methods, optics, etc., can solve problems such as low refractive index, small point anisotropy, and low clearing point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The synthetic route for preparing compound I-4-5 is shown below,
[0068]
[0069] Its specific process steps are as follows:
[0070]
[0071] 1) Add 17mmol of compound A, 17mmol of compound B, 50ml of toluene, 25ml of ethanol, 25ml of water, 68mmol of sodium carbonate into a 250ml three-necked flask, and add 0.85mmol of Pd(PPh 3 ) 4 , continue to reflux under the protection of nitrogen, and react for 6 hours. After the reaction is completed, the reaction solution is post-treated and purified by column chromatography to obtain a white solid I-4-5, GC ≥ 99%.
[0072] R 2 : H, the compound of following structural formula is prepared:
[0073]
[0074] Yield: 86.3%; DSC: C118.2I; △n: 0.183; △ε: 22; Cp: 14.6;
[0075] R 2 :CH 3 , to prepare compounds of the following formula:
[0076]
[0077] Yield: 84.5%; DSC: C106.8I; △n: 0.193; △ε: 24; Cp: 66.6;
[0078] R 2 :C 2 H 5 , to prepare compounds of the following formula:
[0079]
[0080] Yield: 75...
Embodiment 2
[0089] The synthetic route for preparing compound I-4-1 is shown below,
[0090]
[0091] Its specific process steps are as follows:
[0092]
[0093] 1) Add 17mmol of compound A to a 250ml three-necked bottle 1 , 17mmol compound B, 50ml toluene, 25ml ethanol, 25ml water, 68mmol sodium carbonate, under nitrogen protection, add 0.85mmol Pd (PPh 3 ) 4 , continue to reflux under the protection of nitrogen, and react for 6 hours. After the reaction is completed, the reaction solution is post-treated and purified by column chromatography to obtain a white solid I-4-1, GC ≥ 99%.
[0094] R 1 : CH 3 , the compound of the following structural formula is prepared:
[0095]
[0096] Yield: 48%; DSC: C107.3I; Δn: 0.176; Δε: 18.5; Cp: 23.9.
[0097] Compound I-4-1-a 1 H-NMR chart see Figure 5 .
Embodiment 3
[0099] The synthetic route for preparing compound I-6-5 is shown below,
[0100]
[0101] The specific process steps are as follows:
[0102]
[0103] 1) Synthesis of compound D
[0104] In the 250ml there-necked flask, add 20mmol of compound A, 21mmol of 3,5-difluorobromobenzene, 50ml of toluene, 25ml of ethanol, 25ml of water, 80mmol of sodium carbonate, under nitrogen protection, add 1mmol of Pd (PPh 3 ) 4 , continued to reflux under nitrogen protection, and reacted for 6 hours. After the reaction was completed, the reaction solution was post-treated and purified by column chromatography to obtain white solid D with a yield of 75% and GC≥98%.
[0105] 2) Synthesis of compound E
[0106] Add 15mmol of compound D and 100ml of anhydrous tetrahydrofuran to a 250ml three-necked flask, under nitrogen protection, cool down to -78°C, add 4.4ml of n-BuLi solution (2.5mol / L, n-hexane solution) dropwise, after the dropwise addition, continue to control. Stir at -78°C for 1h,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com