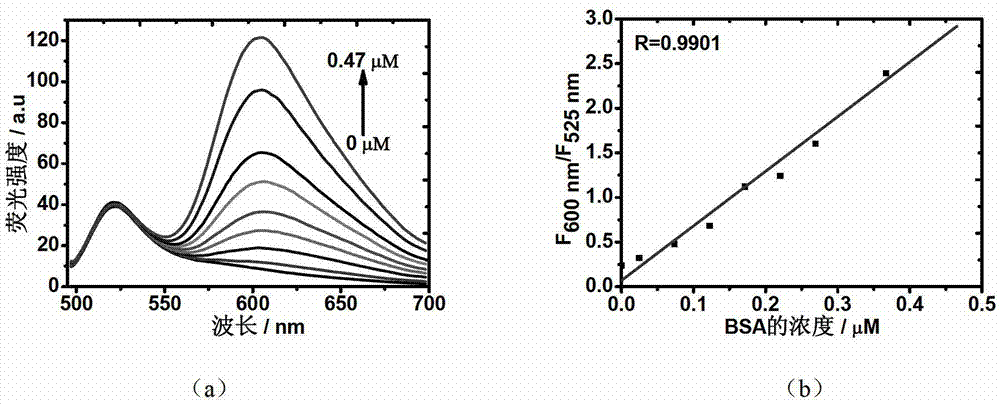
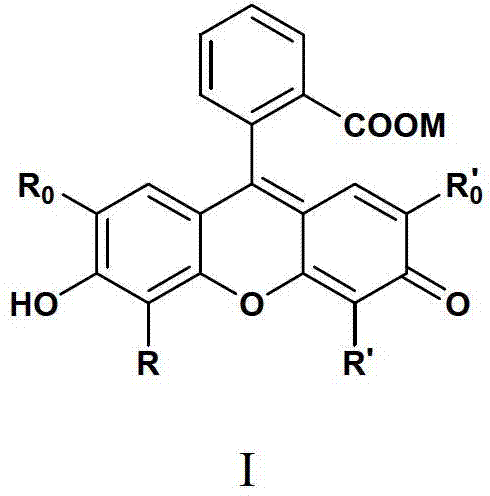
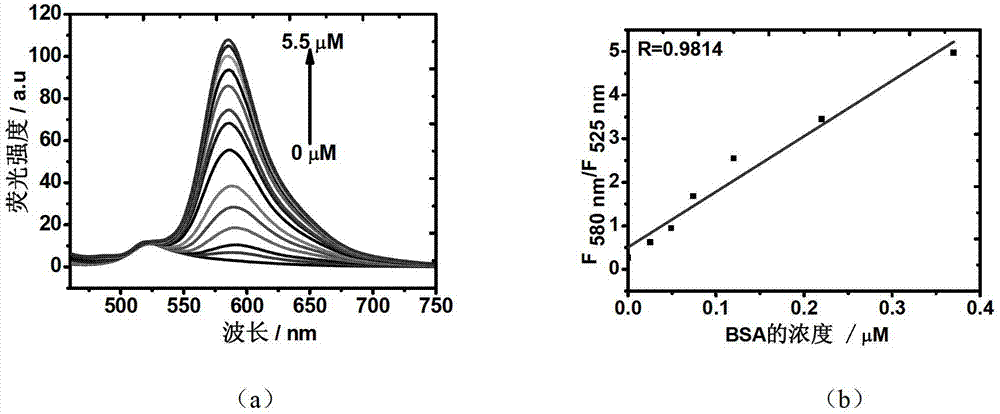
Fluorochrome taking fluorescein as matrix, as well as preparation method and application thereof
A fluorescent dye and fluorescein technology, which is applied to a class of fluorescent dyes with fluorescein as the parent, its preparation and application fields, can solve the problems of prolonging the absorption and emission spectra, and achieve good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] Another aspect of the present invention provides the preparation method of the fluorescent dye with fluorescein as the parent, the method comprising the following steps: preparing the intermediate binary aldehyde respectively, and then combining the intermediate with compounds i, ii, iii, vi, v The compound is obtained by reacting under the action of an organic base (such as piperidine), and the specific synthesis scheme includes the following steps:
[0044] 1) The compound of formula II and the compound of urotropine are reacted at 10-180° C. for 4-48 hours according to the feeding molar ratio of 1:1-1:20, to prepare the compound of formula III;
[0045]
[0046] The reaction solvent is trifluoroacetic acid, dichloromethane, chloroform, ethanol, acetonitrile, ethyl acetate, toluene, xylene, o-dichlorobenzene or a mixture thereof;
[0047] In a preferred embodiment, the reaction temperature is 70-140°C, the reaction time is 2-36 hours, and the reaction solvent is se...
Embodiment 1
[0081] Preparation of probe compound A 1
[0082]
[0083] (1) Synthesis of intermediate 1
[0084] Dissolve DCF (6.30 g, 15.75 mmol, synthesized according to literature), urotropine (10.5 g, 75.1 mmol) in a round bottom flask containing 25 mL of trifluoroacetic acid. This mixture was stirred, heated to 90°C, and stirring was continued for 24h. The mixture became viscous and was treated with aqueous acetic acid (10 mL acetic acid dissolved in 200 mL water). The mixture was then stirred overnight at ambient temperature. A solid precipitated, which was then filtered using a Buchner funnel, and the precipitate was washed 3 times with water. After vacuuming, it was dried under an infrared lamp to obtain a red solid (8.10 g).
[0085] (2) Probe Compound A 1 Synthesis
[0086] Under nitrogen atmosphere, 2.5 mmol of piperidine (0.215 g) was added into absolute ethanol (50 mL) dissolved with DCF dihydric aldehyde (0.32 g, 0.67 mmol) and 2-methylbenzothiazole (339 μL, 2.66 mm...
Embodiment 2
[0088] Preparation of probe compound A 2 :
[0089]
[0090] (1) Synthesis of intermediate 1
[0091] Dissolve DCF (6.30 g, 15.75 mmol, synthesized according to literature), urotropine (10.5 g, 75.1 mmol) in a round bottom flask containing 25 mL of trifluoroacetic acid. This mixture was stirred, heated to 90°C, and stirring was continued for 24h. The mixture became viscous and was treated with aqueous acetic acid (10 mL acetic acid dissolved in 200 mL water). The mixture was then stirred overnight at ambient temperature. A solid precipitated, which was then filtered using a Buchner funnel, and the precipitate was washed 3 times with water. After vacuuming, it was dried under an infrared lamp to obtain a red solid (8.10 g).
[0092] (2) Probe Compound A 2 Synthesis
[0093] Under a nitrogen atmosphere, 2.5 mmol of piperidine (0.215 g) was added into a solution of DCF dihydric aldehyde (0.828 g, 1.82 mmol) and 2,6-dimethyl-4-pyranylidene malononitrile (0.828 g , 1.82 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com