Oral solid preparation taking prucalopride succinate as active ingredient and application of oral solid preparation
A technology of prucalopride succinate and solid preparation, which is applied in the field of medicine and can solve the problem of no 5-HT2 or 5-HT3 receptor antagonistic properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
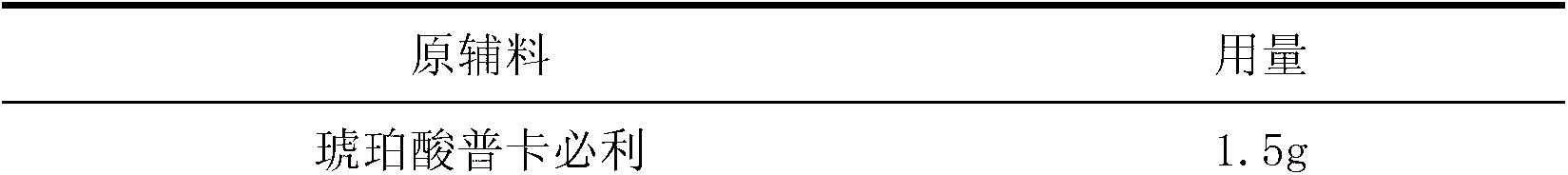
[0016] Make 1000 prucalopride succinate tablets with the raw materials of following weight ratio.
[0017]
[0018]
[0019] Preparation Process:
[0020] 1. The prucalopride succinate raw material is crushed through an 80-mesh sieve and set aside.
[0021] 2. Pass the lactose through a 60-mesh sieve and set aside.
[0022] 3. Take prucalopride succinate and mix well with microcrystalline cellulose, lactose and croscarmellose sodium.
[0023] 4. Make soft material with water, granulate with 20 mesh sieve, dry at 60°C, and granulate with 24 mesh sieve.
[0024] 5. Add magnesium stearate, mix well, and press into tablets.
Embodiment 2
[0026] 1. Prepare 6% ethanol solution of Opadry enteric coating agent;
[0027] 2. Take the plain tablet obtained in Example 1, pour it into the coating pan, start the coating pan, and blow hot air, preheat the plain tablet at 30-40°C for 10 minutes, and blow off the drug powder adhering to the tablet core, Spray the coating solution evenly, so that the drug solution is evenly coated on the tablet core, and then prucalopride succinate enteric-coated tablets are obtained.
Embodiment 3
[0029] Make 1000 prucalopride succinate tablets with the raw materials of following weight ratio.
[0030]
[0031] Preparation:
[0032] 1. Pulverize the prucalopride succinate bulk drug through an 80-mesh sieve and set aside.
[0033] 2. Fully mix prucalopride succinate with sorbitol, microcrystalline cellulose, croscarmellose sodium, and hypromellose E3 evenly.
[0034] 3. Make soft materials with water, granulate with 24 mesh, dry at 60°C, and granulate with 40 mesh.
[0035] 4. Add magnesium stearate, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com