Composition of oral solid medicine having good vivo behaviors
A kind of technology of composition and medicine, applied in the field of oral solid pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: prepare pharmaceutical composition (tablet) of the present invention
[0060] prescription:
[0061]
[0062] Preparation method: pass the prescription amount of active ingredients, methylcellulose K100, microcrystalline cellulose 60mg, and glyceryl behenate 888ATO through a 60-mesh sieve, and mix them evenly in a mixer; then mix xanthan gum with an appropriate amount of water Dissolve as a binder, granulate with the above materials, dry, pass the dry granules through a 20-mesh sieve; then pass another part of microcrystalline cellulose, silicon dioxide 244FP, and talcum powder through a 60-mesh sieve, mix with the above-mentioned dry granules, and press into tablets .
Embodiment 2
[0063] Embodiment 2: prepare pharmaceutical composition (tablet) of the present invention
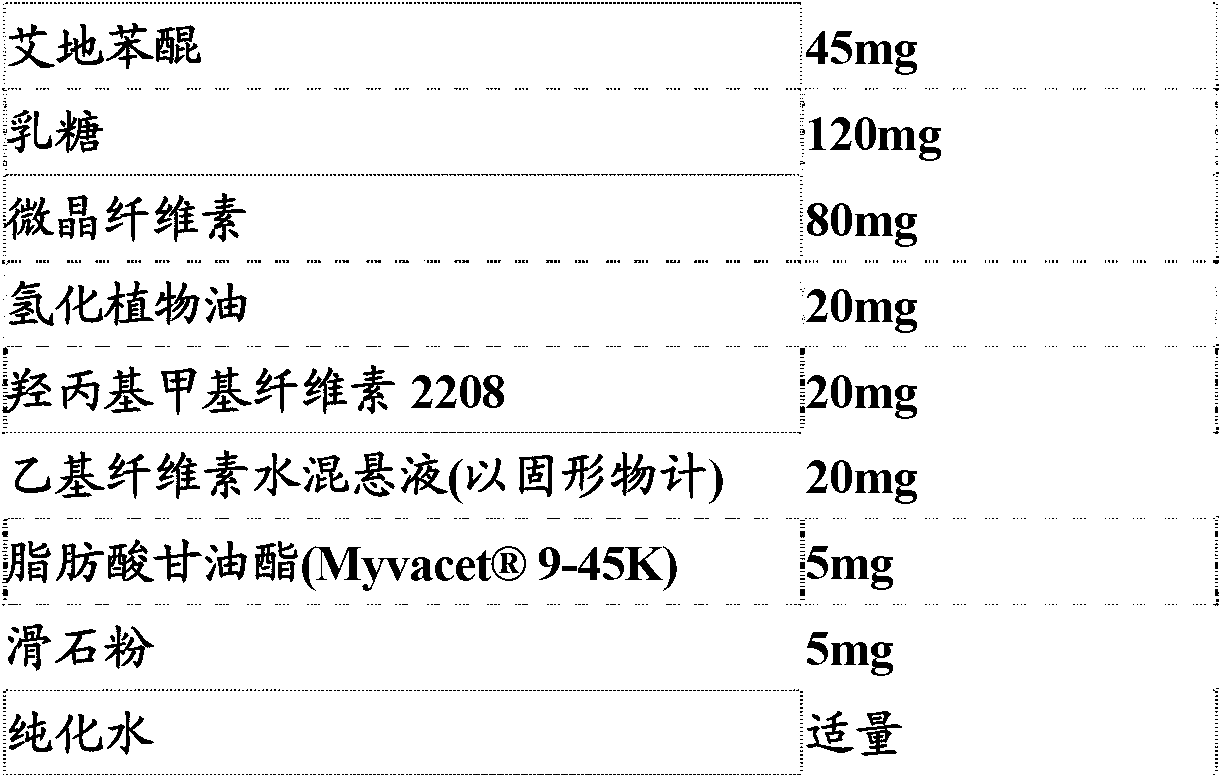
[0064] prescription:
[0065]
[0066] Preparation method: mix the active ingredient, lactose, microcrystalline cellulose, hydrogenated vegetable oil, hydroxypropyl methylcellulose 2208 and fatty acid glycerides in the prescribed amount, and then add ethyl cellulose aqueous suspension (solid content 6 %), mix evenly, granulate, dry, pass the dry granules through a 20-mesh sieve, add talcum powder, mix evenly, press into tablets, and you get it.
Embodiment 3
[0067] Embodiment 3: prepare pharmaceutical composition (tablet) of the present invention
[0068] prescription:
[0069]
[0070] Preparation method: mix the active ingredient, starch, microcrystalline cellulose and hydroxypropyl methylcellulose in the prescribed amount evenly, granulate with a binder, dry, pass the dry granules through a 20-mesh sieve, add magnesium stearate and mix evenly , pressed into tablets, ready to use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com