Method for measuring content of heparin in human antithrombase-III concentrate
An antithrombin and concentrate technology, applied in the field of biological detection, can solve the problems of large interference of antithrombin-III and low measurement accuracy, and achieve the effects of high sensitivity, small reagent dosage and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
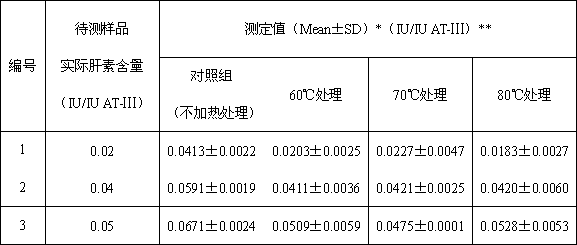
[0028] Example 1: Determination of the content of heparin in antithrombin-Ⅲ solution (heat treatment at different temperatures)
[0029] 1. Processing of samples to be tested:
[0030] (1) Preparation of samples to be tested: take human antithrombin-Ⅲ and heparin standard products, prepare three kinds of human antithrombin-Ⅲ solutions containing different concentrations of heparin, and the concentrations of human antithrombin-Ⅲ are 1IU / ml, the heparin content is 0.02, 0.04 and 0.05IU / ml respectively;
[0031] (2) Divide the three prepared samples into four groups: control group, 60°C heat treatment group, 70°C heat treatment group and 80°C heat treatment group. The solution in the control group was not treated, and the solutions in the three heat treatment groups were incubated at 60°C, 70°C and 80°C for 30 minutes.
[0032] 2. Preparation of standard curve and determination of samples:
[0033] (1) Dilute the heparin standard with known potency to 0.0125, 0.025, 0.05, 0.1...
Embodiment 2
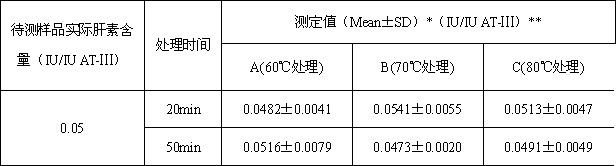
[0051] Example 2: Determination of the content of heparin in antithrombin-Ⅲ solution (using different heat treatment time)
[0052] 1. Processing of samples to be tested:
[0053] (1) Preparation of samples to be tested: take human antithrombin-Ⅲ and heparin standard products, and prepare human antithrombin-Ⅲ solution containing heparin, in which the concentration of human antithrombin-Ⅲ is 1IU / ml. Heparin content is 0.05IU / ml;
[0054] (2) Divide the prepared sample to be tested into two parts, and divide them into three groups A, B and C respectively. C) Incubate under three different temperature conditions for 20 min; the three groups in the second copy were incubated under three different temperature conditions of 60°C (A), 70°C (B) and 80°C (C) for 50 min.
[0055] 2. Preparation of standard curve and determination of samples:
[0056] (1) Dilute the heparin standard with known potency to 0, 0.025, 0.05, 0.1IU / ml with Tris buffer (pH8.4) as the standard solution;
...
Embodiment 3
[0074] Example 3: Determination of Heparin Content in Antithrombin-Ⅲ Concentrate
[0075] 1. Processing of samples to be tested:
[0076] Preparation of the sample to be tested: take the human antithrombin-Ⅲ concentrate purified by heparin affinity chromatography, its concentration is determined according to the method of the 32nd edition of the United States Pharmacopoeia, and accurately diluted to 1IU / ml, the diluted sample Divide into three parts and incubate for 30 min at three different temperature conditions of 60°C, 70°C and 80°C respectively.
[0077] 2. Preparation of standard curve and determination of samples:
[0078] (1) Dilute the heparin standard with known potency to 0, 0.0125, 0.025, 0.05, 0.1IU / ml with Tris buffer (pH8.4) as the standard solution;
[0079] (2) Take 40 μl of heparin standard solution and three kinds of sample solutions to be tested in different wells of the microplate;
[0080] (3) Add an equal volume of 1IU / ml antithrombin-Ⅲ standard sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com