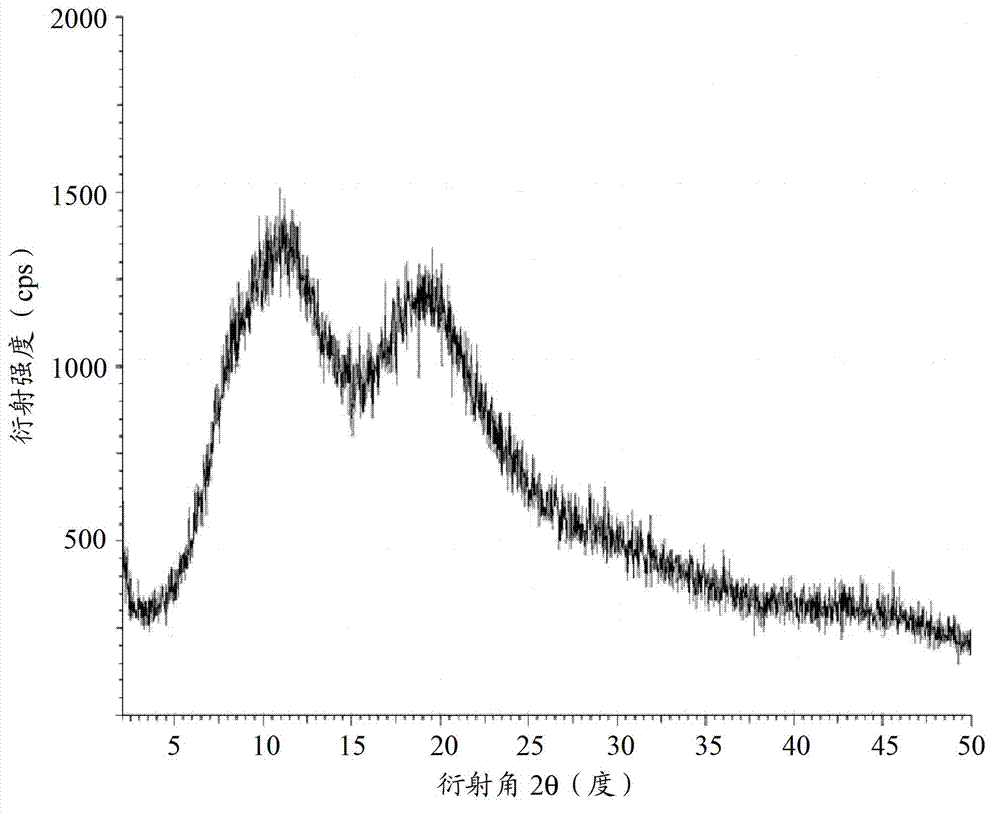
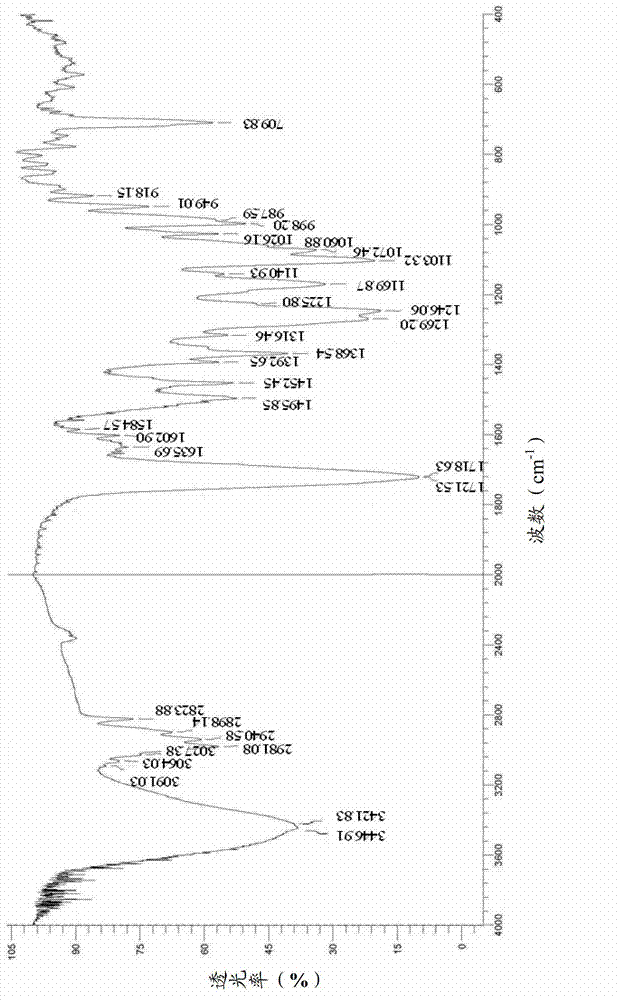
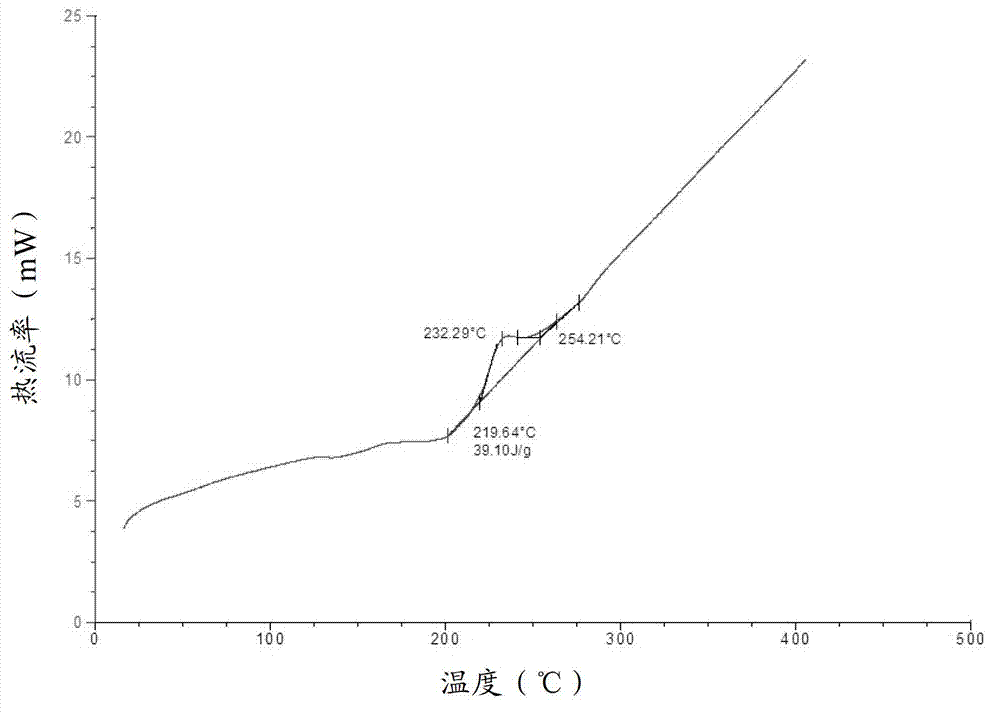
Cabazitaxel amorphous crystal and preparation method thereof
A cabazitaxel, amorphous technology, applied in the field of the crystal form of cabazitaxel and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Embodiment 1 Preparation of Cabazitaxel Amorphous Crystal
[0158] Add 5 g of cabazitaxel anhydrous crystals into 75 mL of ethanol, stir and dissolve, concentrate to dryness at 50°C to obtain cabazitaxel powder, and dry the cabazitaxel powder at 60°C under reduced pressure for 12 hours to obtain 4.9 g of cabazitaxel Race amorphous crystal. The purity detected by HPLC is ≥99.87%, the residue of ethanol detected by GC is 1608ppm, and the moisture is 0.9%.
Embodiment 2
[0159] Embodiment 2 Preparation of Cabazitaxel Amorphous Crystal
[0160] Dissolve 10 g of cabazitaxel hydrate crystals in 100 mL of dichloromethane and concentrate to dryness at 30°C to obtain cabazitaxel powder. Dry the cabazitaxel powder under vacuum at a temperature of 50-60°C to obtain 9.9g Cabazitaxel amorphous crystal. The purity detected by HPLC is ≥99.84%, the residual dichloromethane detected by GC is 13080ppm, and the water content is 0.5%.
Embodiment 3
[0161] Example 3 Preparation of Cabazitaxel Amorphous Crystal
[0162] Dissolve 10 g of cabazitaxel solvate crystals in 250 mL of acetone, then concentrate to dryness at 40°C to obtain cabazitaxel powder, and vacuum-dry the cabazitaxel powder at a temperature of 50-60°C to obtain 9.9 g of cabazitaxel He is an amorphous crystal. The purity detected by HPLC is ≥99.84%, the residual acetone detected by GC is 8562ppm, and the water content is 0.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com