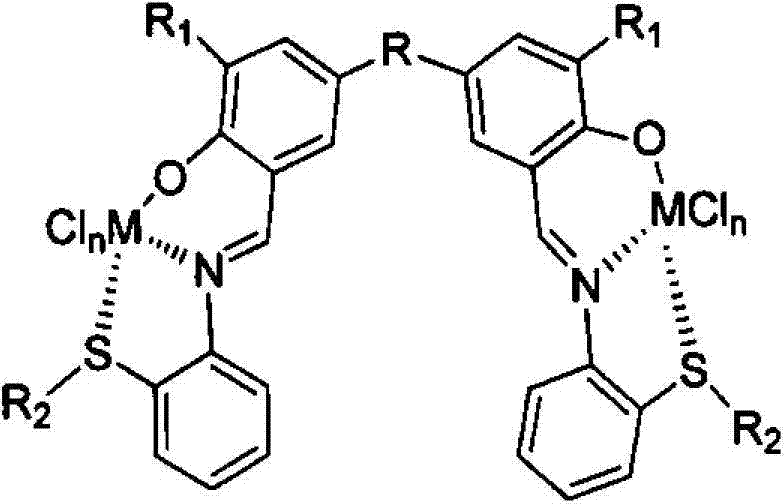
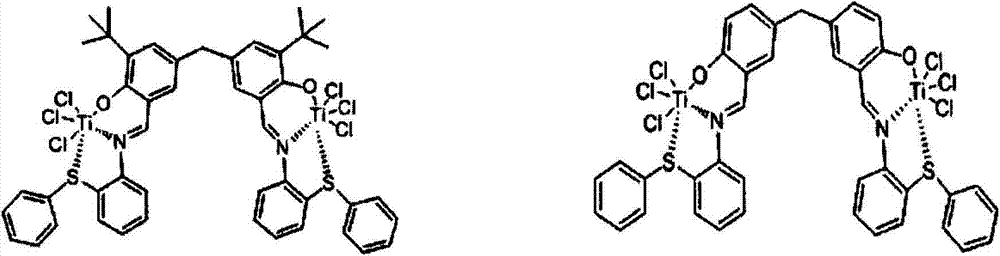
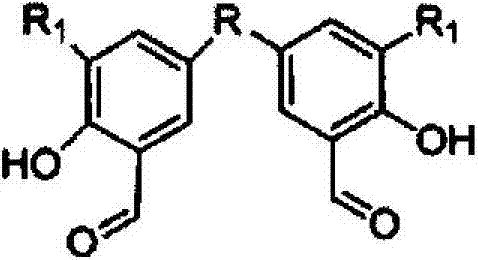
ONS (Organometallics) type salicylaldimine binuclear metallic alkene catalyst and preparation method thereof
A technology of salicylaldimine and olefin catalyst, applied in the field of olefin coordination polymerization, can solve the problems of poor tolerance of polar monomers, single active center, difficult resin processing, etc., and achieve the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The preparation method of ONS type salicylaldimine binuclear metal olefin catalyst comprises the following steps:
[0050] (1) Preparation of dual-nuclear structure salicylaldehyde or salicylaldehyde derivatives: under an inert atmosphere, mix salicylaldehyde or salicylaldehyde derivatives, glacial acetic acid, and reactants for bridging in a ratio of 0.1-1.0 / mol : 10-100 / ml: 0.05-0.5 / ml, under the action of concentrated sulfuric acid, react at 90-95°C for 24h to obtain binuclear structure salicylaldehyde or salicylaldehyde derivatives;
[0051] The molecular structural formula of the prepared binuclear structure salicylaldehyde or salicylaldehyde derivative is as follows:
[0052]
[0053] (2) Preparation of Schiff's base: under an inert atmosphere, the binuclear structure salicylaldehyde or salicylaldehyde derivatives, amine, and ethanol are prepared in a ratio of 0.1-1 / mol: 0.15-2.0 / ml: 0.01-0.05 / ml: 50-200 / ml, under the catalysis of a small amount of acid, the c...
Embodiment 1
[0069] 3-tert-butylsalicylaldehyde was synthesized from o-tert-butylphenol as the starting material under the catalysis of anhydrous tin tetrachloride. Then under the effect of the concentrated sulfuric acid, obtain 5,5'-methylene-bis-3-tert-butyl salicylaldehyde with trioxane as the bridging reactant. Under the action of p-toluenesulfonic acid, 2-aminodiphenylphenyl sulfide is added to react with the binuclear skeleton to obtain the Schiff base. Schiff base and TiCl 4 Complexation obtains an ONS type binuclear metal olefin polymerization catalyst, and the specific implementation steps are as follows:
[0070] 1) Preparation of 3-tert-butyl salicylaldehyde: under the protection of nitrogen, add 25g of tert-tert-butylphenol, 12.5g of 2,6-lutidine, 200ml of anhydrous toluene to the dry reaction bottle, then drop 2ml After the addition of anhydrous tin tetrachloride was completed, the solution was stirred at room temperature for 0.5 h, and then 10 g of paraformaldehyde was adde...
Embodiment 2
[0075] Use the ONS type binuclear olefin polymerization catalyst prepared in Example 1 to carry out ethylene polymerization: under 1 atm ethylene atmosphere, add successively 13 ml of toluene solution that is dissolved with 3.01 mg of catalyst, 12 mmol of MAO, and 27 ml of anhydrous toluene in a dry polymerization reaction bottle , the catalyst concentration was 0.075 μmol / ml. Stir the reaction at 10°C for 1 h, pour the reactant into 300 ml of 0.5% ethanol hydrochloric acid solution by volume, filter, wash twice with ethanol hydrochloric acid solution of 0.5% by volume, wash three times with ethanol, and vacuum After drying, 0.94 g of polyethylene was obtained. The catalytic activity is 0.16×10 6 gPE·mol -1 Ti·h -1 . The weight average molecular weight of polyethylene M w 3.0×10 5 , The molecular weight distribution index PDI is 2.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com