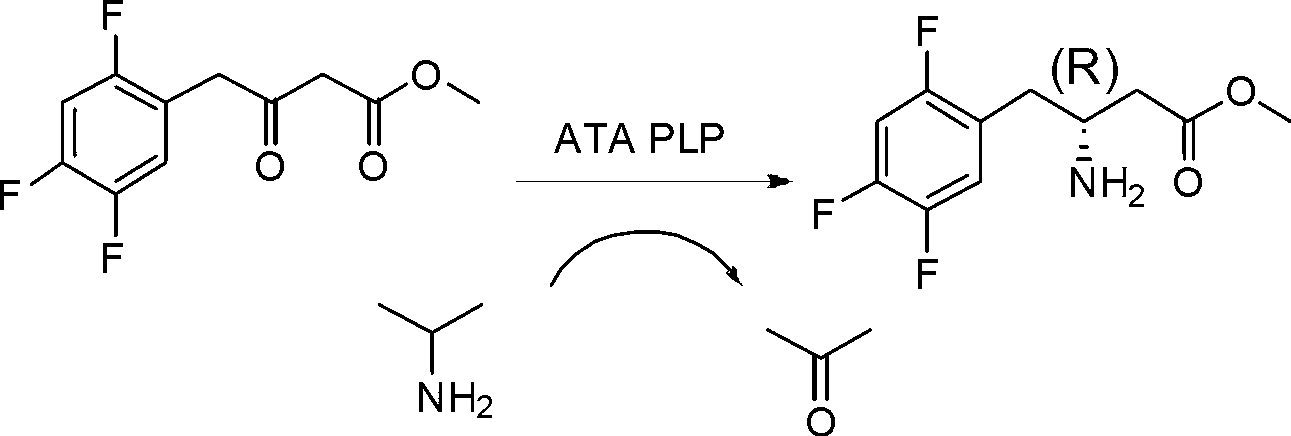
Biological preparation method of 3-amino-4-(2,4,5-trifluorophenyl)methyl butyrate
A technology of trifluorophenyl and methyl butyrate, applied in the fields of biopharmaceuticals and green chemistry, to achieve the effects of less by-products, simple and convenient operation, and high optical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Single colonies from glycerol tubes or transformation plates were inoculated into 4 mL of liquid LB medium containing kanamycin resistance for overnight activation (37°C, 200 rpm), and the inoculum of 1 / 100 (v / v) was transferred to 100mL of liquid LB medium containing kanamycin resistance, 37°C, 200rpm shaking culture until the OD600 value reaches 0.6-0.8, add IPTG and continue culturing at 30°C overnight, centrifuge to collect cells, wash with 10mL phosphate buffer (2mM, pH7 .0) Suspend the cells, place the cell suspension in an ice bath for 10 minutes by ultrasonication, then centrifuge, pre-freeze the supernatant overnight to a temperature of -10~-25°C, and freeze-dry for 24h-48h.
Embodiment 2
[0026] In a 20mL three-necked flask, add 3.85mL of 0.1M pH 7.0 phosphate buffer solution, 100mg of substrate, 900μL of isopropylamine, 0.25mL of DMSO, 1mg of pyridoxal phosphate (PLP), 50mg of transaminase, at 30°C, 200rpm stirring paddle Stirring, under the condition of 0.01MPa nitrogen purging, reacted for 24h, and the conversion rate was detected by HPLC>95%. Add hydrochloric acid to adjust the pH to 2-3, filter with celite, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain 90 mg of the product with a purity of >95% and an optical purity of >99%.
Embodiment 3
[0028] In the reactor, add 3.85L of 0.1M pH 7.0 phosphate buffer solution, 100g of substrate, 900mL of isopropylamine, 0.25L of DMSO, 1g of pyridoxal phosphate (PLP), 50g of transaminase, at 30°C, mechanical stirring paddle Stirring, under the condition of 0.01MPa nitrogen purging, reacted for 24h, and the conversion rate was detected by HPLC>95%. Add hydrochloric acid to adjust the pH to 2-3, filter with diatomaceous earth, add an equal volume of ethyl acetate to extract twice, and rotary evaporate to obtain about 90 g of the product, with a purity of >95% and an optical purity of >99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com