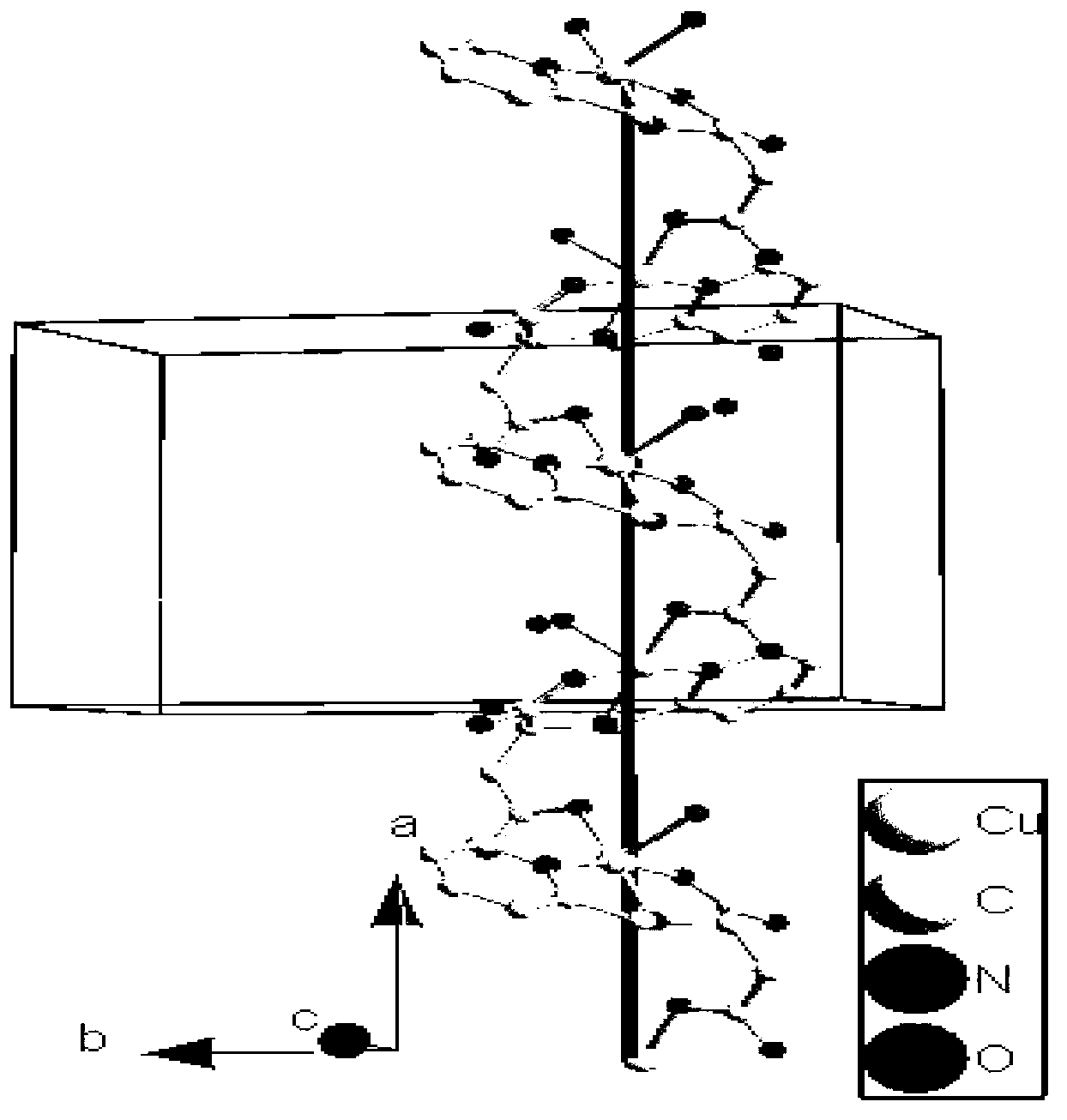
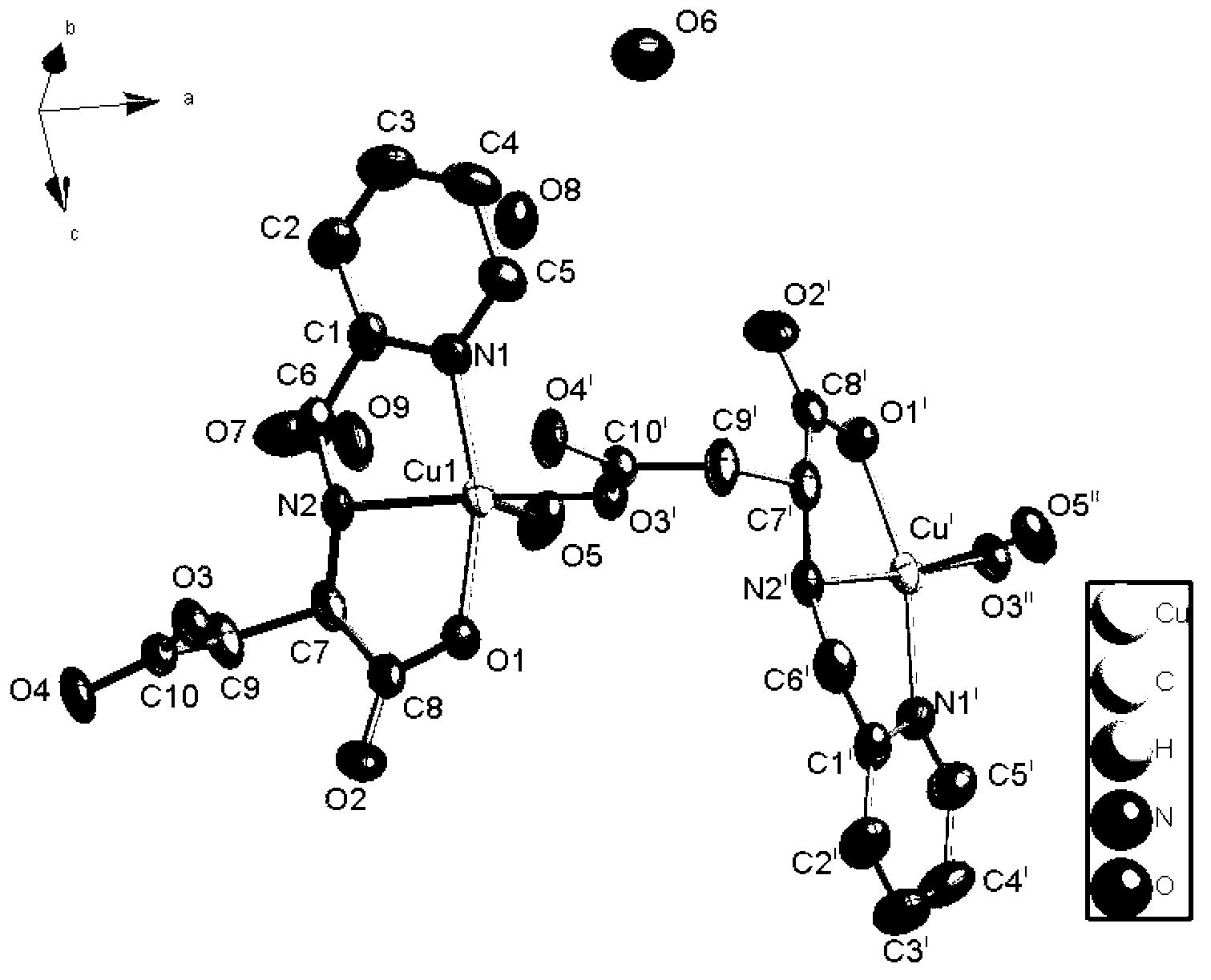
Cuprum (II) coordination compound catalyst of selective catalytic oxidation thioether and preparation method of cuprum (II) coordination compound catalyst
A sulfide oxidation, selective technology, applied in the direction of organic compound/hydride/coordination complex catalyst, organic compound preparation, physical/chemical process catalyst, etc., can solve the problem of high reaction temperature, long reaction time and harsh conditions. and other problems, to achieve the effect of good thermal stability, simple operation and simple reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0031] Preparation of Experimental Example 1 Complexes I and II:
[0032] 1mmol N-(2-pyridylmethyl)-L(D)-aspartic acid (224mg), 2mmol copper chloride (340mg) were dissolved in 10mL 1,4-dioxane and water (V / V=2 :1) in the mixed solution, wherein the molar ratio of the copper salt and N-(2-pyridylmethyl)-aspartic acid is 2:1. Mix and stand for a period of time, filter, seal the filtrate, and get blue blocky crystals after a few days, which are collected by filtration, washed with ethanol, and dried naturally. The yield is 84% (based on pasp, the same below).
experiment example 2
[0033] Preparation of Experimental Example 2 Complexes I and II:
[0034] 2mmol N-(2-pyridylmethyl)-L(D)-aspartic acid (448mg), 1mmol copper chloride (170mg) were dissolved in 10mL 1,4-dioxane and water (V / V=1 :2) in the mixed solution, wherein the molar ratio of the copper salt and N-(2-pyridylmethyl)-aspartic acid is 1:2. Mix and stand for a period of time, filter, and seal the filtrate to puncture the holes. After a few days, blue blocky crystals are obtained, which are collected by filtration, washed with ethanol, and dried naturally. The yield is 81%.
experiment example 3
[0035] Preparation of Experimental Example 3 Complexes I and II:
[0036] 1mmol N-(2-pyridylmethyl)-L(D)-aspartic acid (224mg), 1mmol copper chloride (170mg) were dissolved in 10mL 1,4-dioxane and water (V / V=1 :1) in the mixed solution, wherein the molar ratio of the copper salt and N-(2-pyridylmethyl)-aspartic acid is 1:1. Mix and let stand for a period of time, filter, seal the filtrate and puncture the holes, obtain blue blocky crystals after a few days, collect them by filtration, wash with ethanol, and dry naturally, the yield is 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com