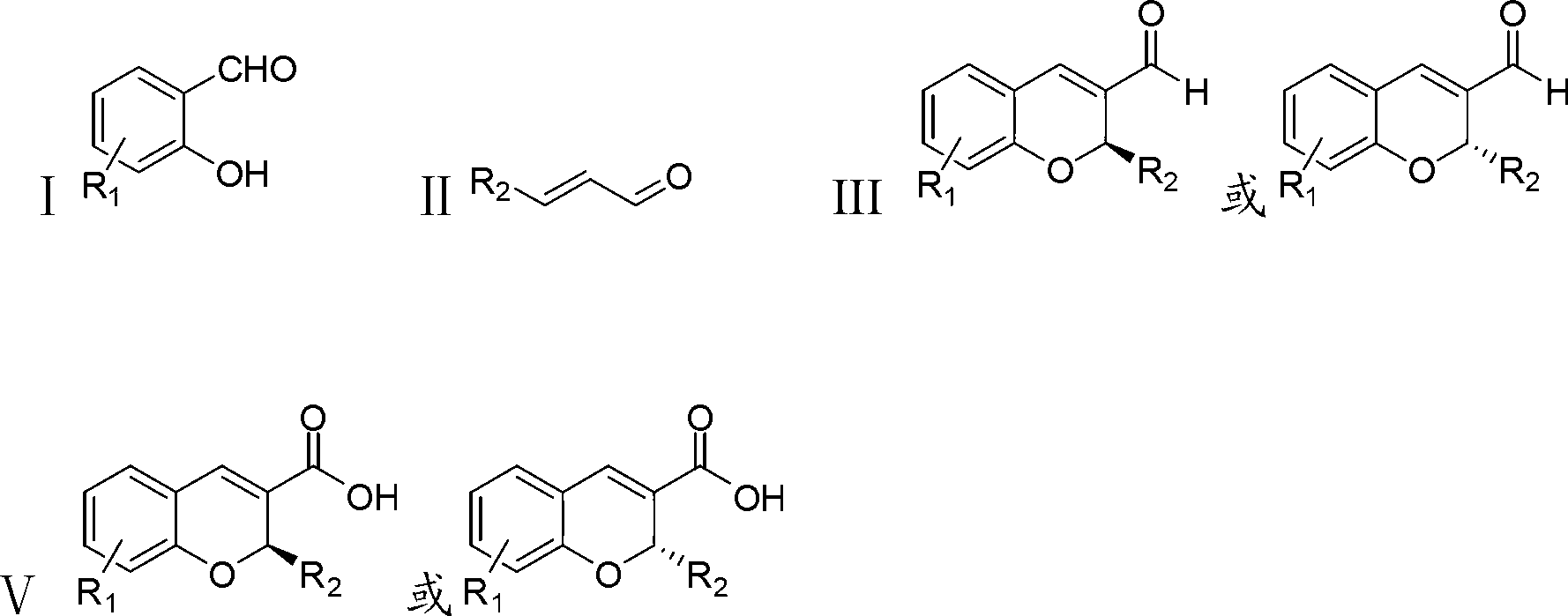
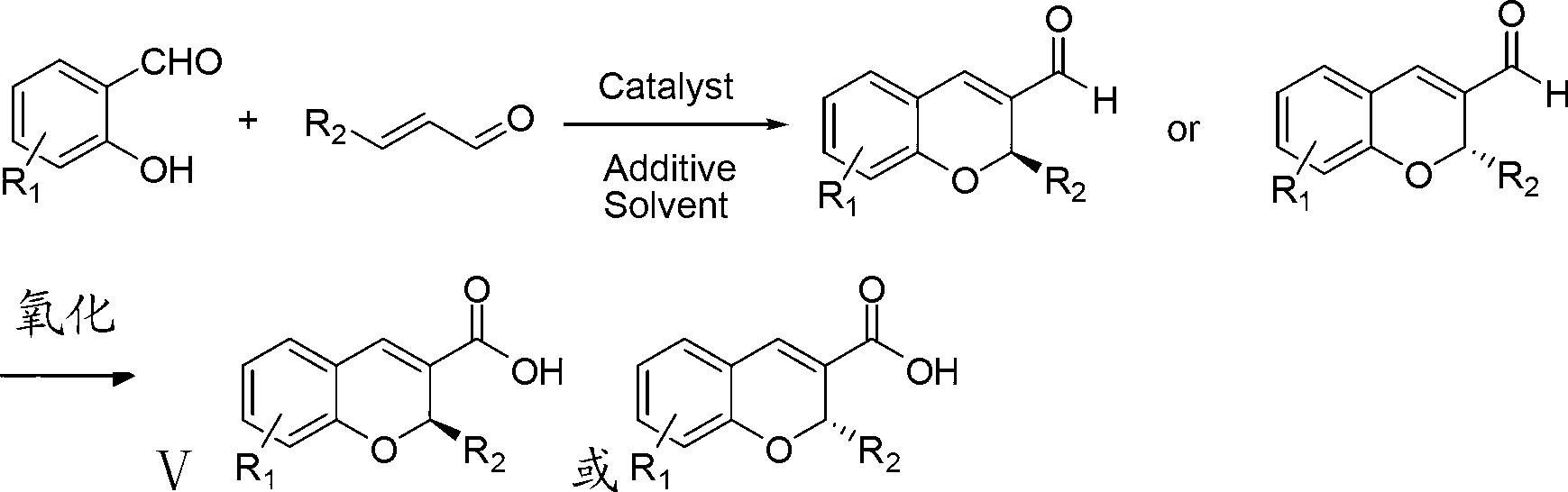
Synthetic method of benzopyran chiral compound
A technology of chiral compounds and benzopyrans, applied in the field of chemistry, can solve the problems of high price, low yield, unfavorable for large-scale application, etc., and achieves easy availability of raw materials, high optical purity, and is conducive to large-scale applications. Effects of production applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
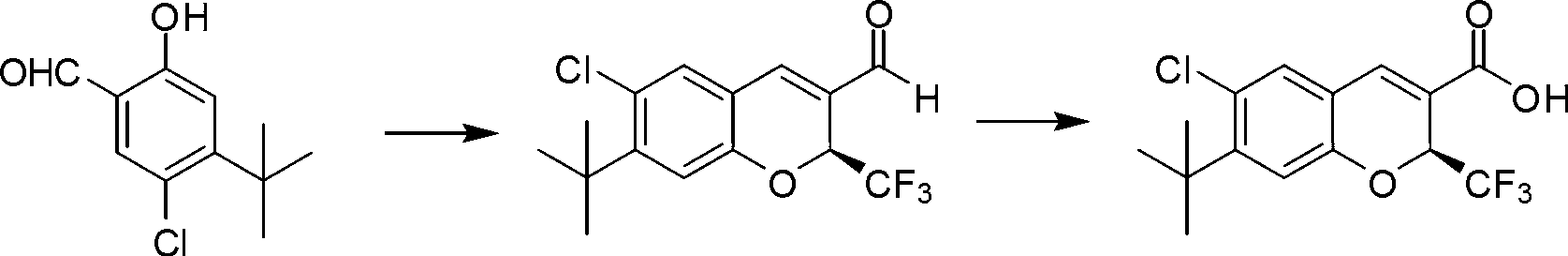
[0045] Synthesis of (S)-7-tert-butyl-6-chloro-2-trifluoromethyl-2H-chromene-3-carboxylic acid
[0046] The synthetic route of (S)-7-tert-butyl-6-chloro-2-trifluoromethyl-2H-chromene-3-carboxylic acid is as follows:
[0047]
[0048] Specific steps are as follows:
[0049] Step 1: Synthesis of (S)-7-tert-butyl-6-chloro-2-trifluoromethyl-2H-benzopyran-3-aldehyde
[0050] The compound 4-tert-butyl-5-chloro-2-hydroxybenzaldehyde (1.0g, 4.7mmol) with the structure of formula I, the compound 4,4,4-trifluorobut-2-enal with the structure of formula II (1.16g, 9.4mmol), chiral catalyst (2S)-2-[diphenyl[(trimethylsilyl)oxy]methyl]-pyrrolidine (0.31g, 0.94mmol), chemical additives Nitrobenzoic acid (0.16 g, 0.94 mmol) was mixed in 50 mL of ethyl acetate. The reaction solution was stirred at 25°C for 24 hours. After the reaction was completed, the product (S)-7-tert-butyl-6-chloro-2-trifluoromethyl-2H-benzopyran-3-aldehyde was obtained by column chromatography (structure as follows...
Embodiment 2
[0063] Synthesis of (S)-6-chloro-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-chromene-3-carboxylic acid
[0064] The synthetic route of (S)-6-chloro-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-chromene-3-carboxylic acid is as follows:
[0065]
[0066] Specific steps are as follows:
[0067] Step 1: Synthesis of (S)-6-chloro-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-benzopyran-3-aldehyde
[0068] The compound 3-chloro-6-hydroxyl-2,4-deuterated dimethylbenzaldehyde (1.0g, 5.2mmol) with the structure of formula I, the compound 4,4,4-trifluorobutanol with the structure of formula II 2-enal (1.3g, 10.4mmol), chiral catalyst (2S)-2-[diphenyl[(trimethylsilyl)oxy]methyl]-pyrrolidine (0.34g, 1.04mmol), Chemical Additions p-Nitrobenzoic acid (0.34 g, 2.08 mmol) was mixed in 50 mL of chloroform. The reaction solution was stirred at 25°C for 48 hours. After the reaction, the product (S)-6-chloro-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-benzo Pyran-3-aldehyde (structure as follows...
Embodiment 3
[0082] Synthesis of (S)-6-bromo-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-chromene-3-carboxylic acid
[0083] The synthetic route of (S)-6-bromo-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-chromene-3-carboxylic acid is as follows:
[0084]
[0085] Specific steps are as follows:
[0086] Step 1: Synthesis of (S)-6-bromo-5,7-deuterodimethyl-2-(trifluoromethyl)-2H-benzopyran-3-aldehyde
[0087] The compound 3-bromo-6-hydroxyl-2,4-deuterodimethylbenzaldehyde (1.0g, 4.3mmol) with the structure of formula I, the compound 4,4,4-trifluorobutanol with the structure of formula II 2-enal (1.1g, 8.6mmol), chiral catalyst (2S)-2-[diphenyl[(trimethylsilyl)oxy]methyl]-pyrrolidine (0.28g, 0.86mmol), The chemical additive o-nitrobenzoic acid (0.14g, 0.86mmol) was mixed in 50mL chloroform. The reaction solution was stirred at 25°C for 24 hours. After the reaction, the product (S)-6-bromo-5,7 -Deuterated dimethyl-2-(trifluoromethyl)-2H-benzopyran-3-al (structure as follows) 1.2g (yield: 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com