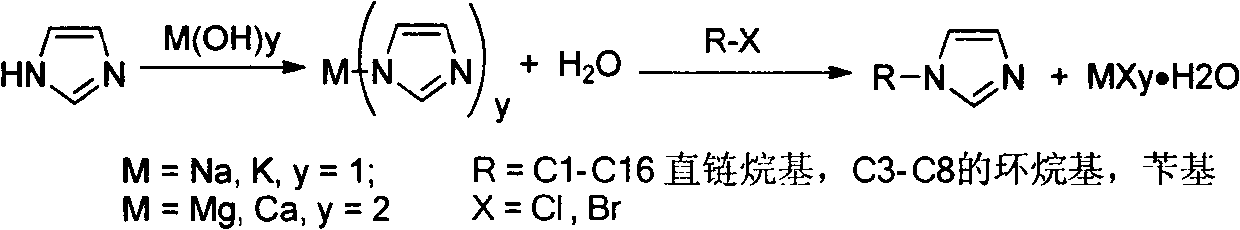
Method for producing high-purity N-alkyl imidazole
A technology for the production of alkylimidazoles and methods, applied in the field of preparation of high-purity N-alkylimidazoles, achieving the effects of high purity, simple process, and increased income
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
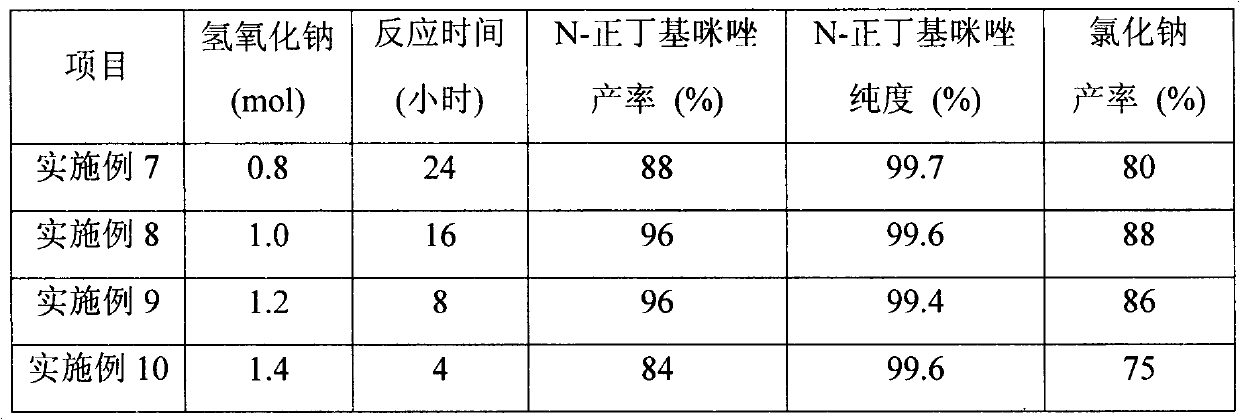
Image
Examples
Embodiment 1
[0030] Take imidazole (68.0g, 1.0mol) and sodium hydroxide (40.0g, 1.0mol) in a 1000mL three-necked flask with a thermometer, stir vigorously at 90°C for 20 hours, then cool down to 24°C, add 300mLTHF, and then add chlorine Substitute n-butane (92.0g, 1.0mol), react at 70°C for 12 hours, filter after the reaction, wash the solid three times with 10% of the original volume of THF, combine the organic phase, and finally distill 285mLTHF under normal pressure for recycling , the remaining liquid was subjected to vacuum distillation (80-82° C., 1 torr), to obtain 105.0 g of high-purity product N-n-butylimidazole, with a yield of 85% and a purity of 99.6%. The obtained NaCl was dissolved in 150 mL of hot water, cooled and crystallized for purification, filtered, and dried to obtain 50 g of white sodium chloride solid with a yield of 85%.
Embodiment 2
[0032] Take imidazole (6.8g, 0.1mol) and sodium hydroxide (4.0g, 0.1mol) in a 1000mL three-necked flask with a thermometer, stir vigorously at 90°C for 20 hours, then cool down to 0°C, add 680mL of THF, and then add olefin Propyl chloride (7.6g, 0.1mol), reacted at 20°C for 12 hours, filtered after the reaction, washed the solid three times with 10% THF of the original volume, combined the organic phase, and finally distilled 696mLTHF under normal pressure for recycling. The remaining liquid was subjected to vacuum distillation (76-78° C., 1 torr) to obtain 9.5 g of high-purity N-allylimidazole with a yield of 88% and a purity of 99.1%. The obtained NaCl was dissolved in 15 mL of hot water, cooled and crystallized for purification, filtered and dried to obtain 5.1 g of white sodium chloride solid with a yield of 87%.
Embodiment 3
[0034] Take imidazole (68.0g, 1.0mol) and sodium hydroxide (40.0g, 1.5mol) in a 250mL three-necked flask with a thermometer, stir vigorously at 150°C for 0.2 hours, then cool down to 0°C, add 68mLTHF, and then add a Methyl bromide (85.5g, 0.8mol), reacted at 20°C for 4 hours, filtered after the reaction, washed the solid three times with 10% of the original volume of THF, combined the organic phase, and finally distilled 60mLTHF under normal pressure for recycling, and the remaining liquid Rectification under reduced pressure (46-48° C., 1 torr) yielded 60.6 g of high-purity N-methylimidazole with a yield of 92% and a purity of 99-8%. The obtained NaCl was dissolved in 150 mL of hot water, cooled and crystallized for purification, filtered, and dried to obtain 47.6 g of white sodium chloride solid, with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com