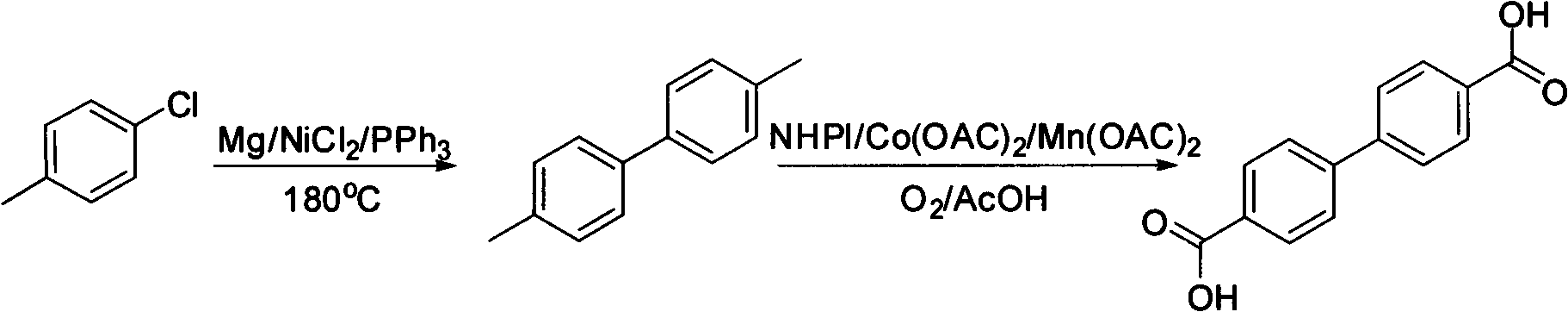
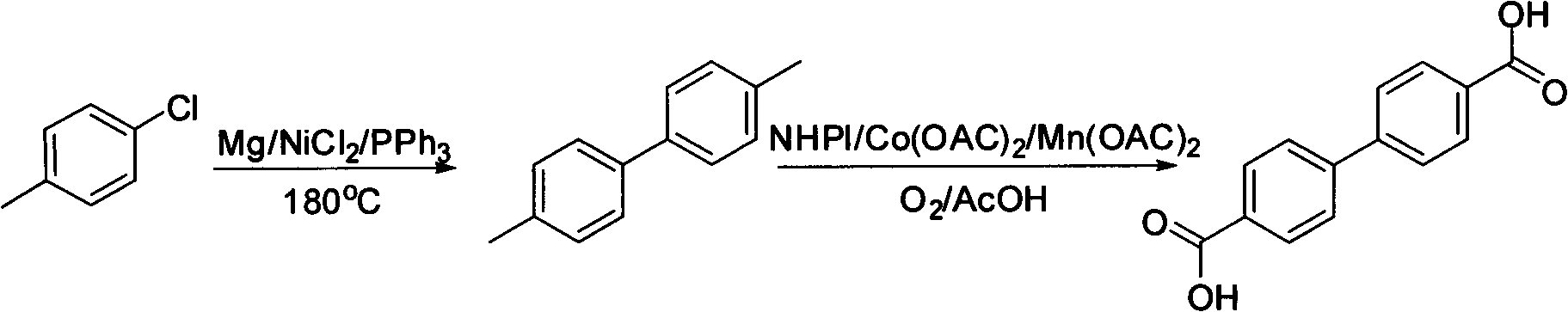
Method for synthesizing biphenyl 4,4'-dicarboxylic acid from p-chlorotoluene
A technology of biphenyl dicarboxylic acid and p-chlorotoluene, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate and other directions, can solve the problems of large environmental pollution, numerous steps, complicated operations, etc., so as to reduce environmental pollution. , The effect of simple and convenient operation and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1) Inert gas (N 2 ) under protection, 0.3g is now filing magnesium powder, 5mL p-chlorotoluene is added in the dry there-necked flask, 81mL NiCl is added 2 , 164mg PPh 3 , add a reflux condensing device, heat to 180 ° C, react for 2.5 h, and cool to room temperature. 2 mL of methanol was added for quenching, 20 mL of ethyl acetate was added for extraction, celite was filtered, washed twice with saturated brine, dried over anhydrous sodium sulfate, evaporated to dryness, and the unreacted p-chlorotoluene was recovered to obtain 1.4 mg of pale yellow solid , the yield is 63%. The crude product has a purity of more than 90%, and is recrystallized with methanol to obtain a white slightly yellow crystalline solid with a HPLC purity of more than 98%. Melting point: 119-121℃
[0028] 2) put 2mmol of the 4,4'-dimethylbiphenyl obtained in step 1) into a dry single-neck bottle, add 0.4mmol of NHPI, 5mg of Co(OAC) 2 , 5mg Mn(OAC) 2 , add 6 mL of acetic acid as a solvent, and...
Embodiment 2
[0030] With embodiment 1, wherein the difference is that the amount of p-chlorotoluene added in step 1) is 4.4mL, and the ligand PPh 3 328mg, the reaction reflux time was 4h, and finally 1.54mg of 4,4'-dimethylbiphenyl was obtained, and the yield was 68.5%.
Embodiment 3
[0032] With embodiment 1, wherein the difference is that the amount of p-chlorotoluene added in step 1) is 5.7mL, and the ligand PPh 3 328mg, the reaction reflux time was 3h, and finally 1.78mg of 4,4'-dimethylbiphenyl was obtained, and the yield was 79%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com