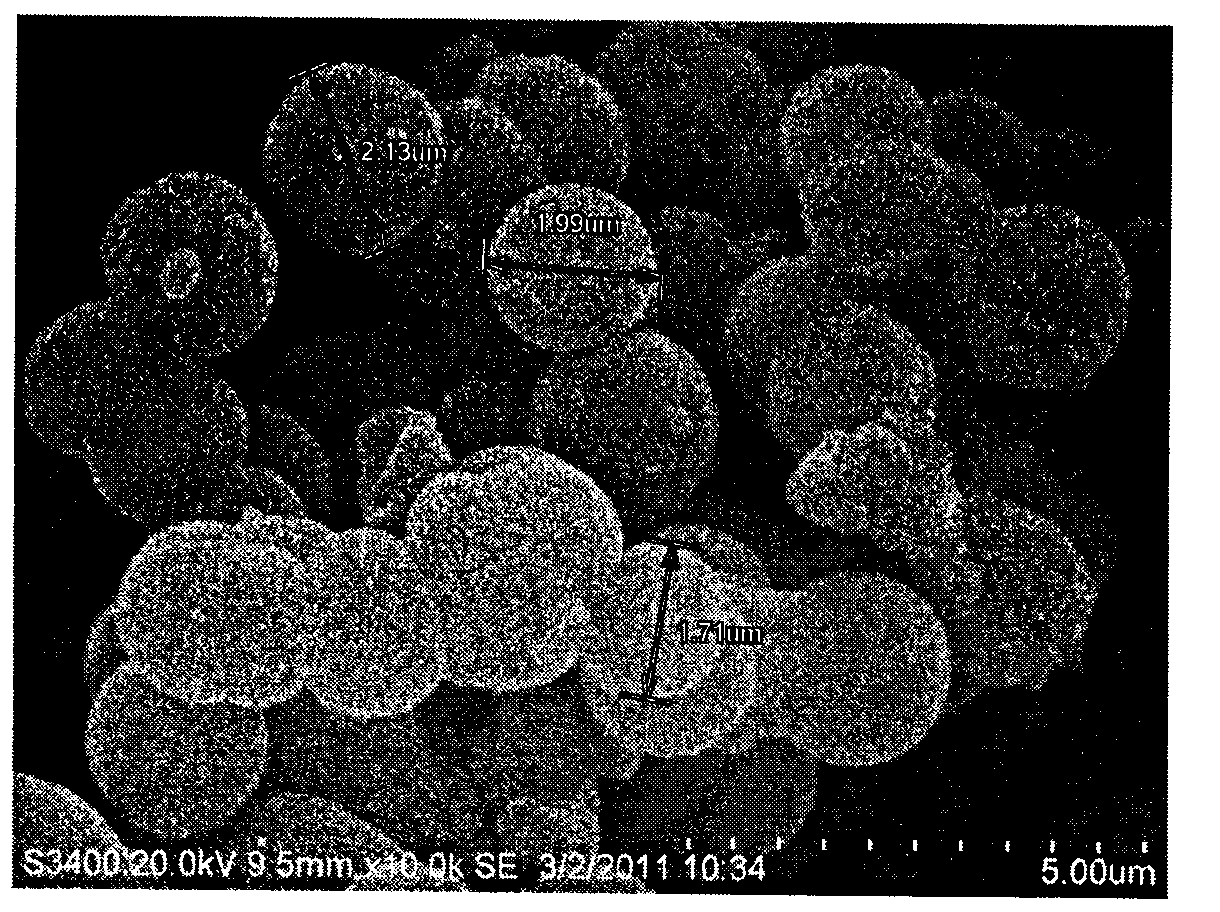New cyclovirobuxine D purifying method
A technology of cyclovir buxicine and a purification method, which is applied in the field of purification of traditional Chinese medicine raw materials, can solve problems such as difficult separation and purification, and achieve the effects of easy commercialization, good separation efficiency, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of mesoporous silica
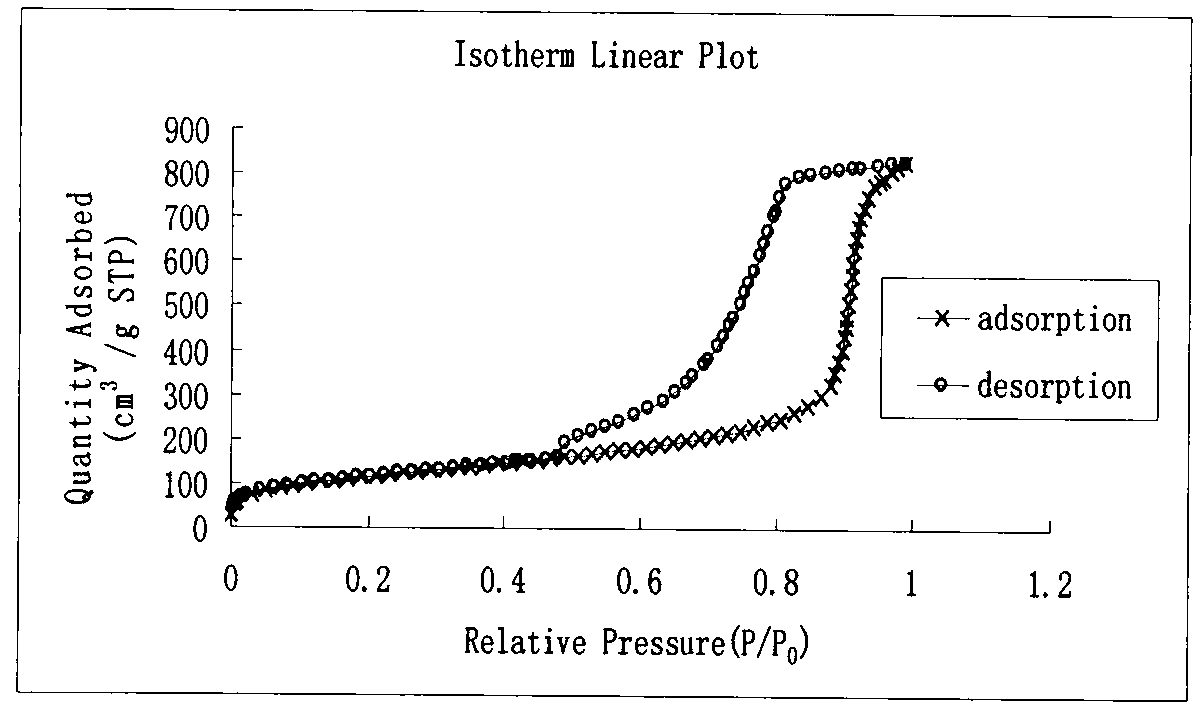
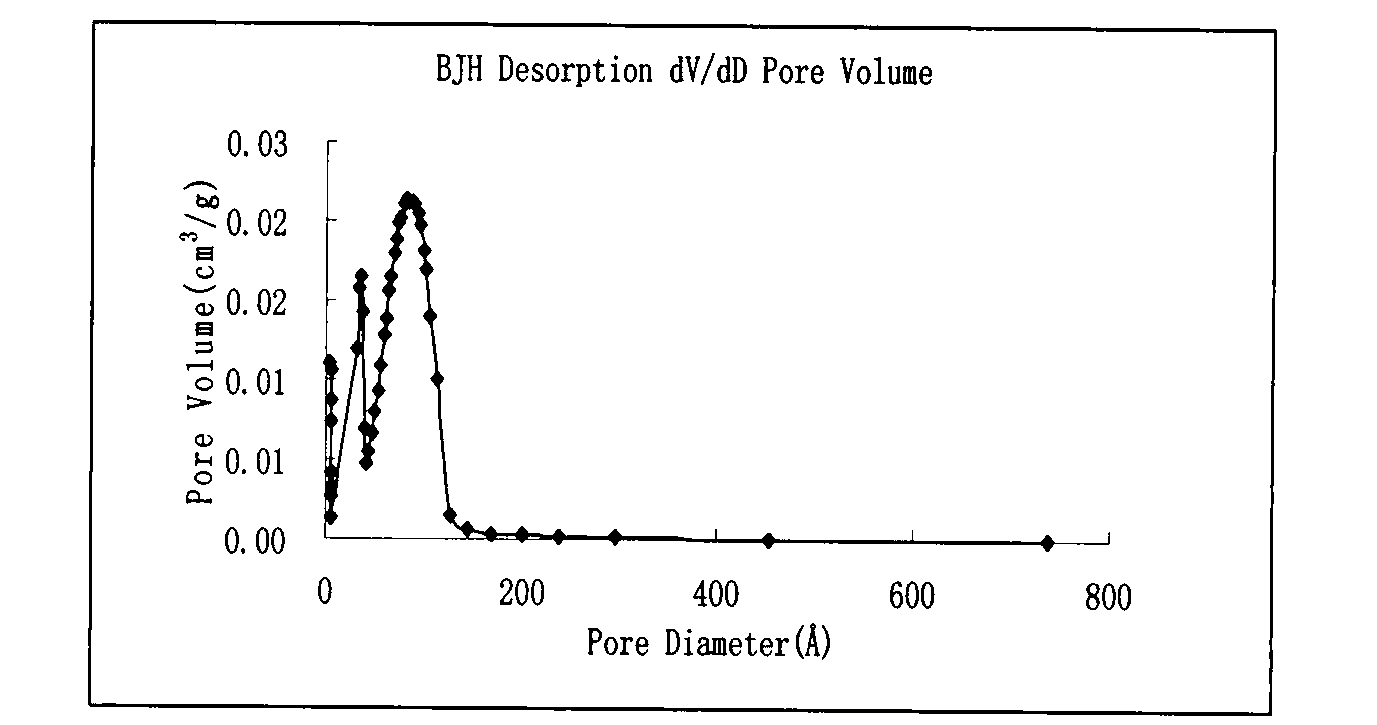
[0027] At room temperature, weigh P1230.5021g and ammonium chloride 0.24g into a 50ml round-bottomed flask, add 15ml of deionized water, 2ml of ethanol and 3ml of concentrated hydrochloric acid in sequence, and stir to mix them evenly to obtain a clear and transparent solution. Under vigorous stirring, 450 ul of 1,3,5-trimethylbenzene was added dropwise, and the mixture was stirred at 35°C for 10 hours. 1150 ul of tetraethoxysilane was added dropwise to the above reaction solution, and vigorously stirred at 35° C. for 15 minutes. The reaction solution was transferred to a stainless steel polytetrafluoro reaction kettle and allowed to stand at 35°C for 24 hours, and then the temperature was raised to 90°C and allowed to stand for 24 hours. Filter while hot, wash with ethanol and deionized water respectively, dry at 90°C under normal pressure for 10 hours, and calcinate at 550°C for 6 hours to remove the template agent. The material has...
Embodiment 2
[0029] Preparation of Mesoporous Silica Surface Imprinting
[0030] Weigh 4.05g of mesoporous silica gel, put it in a 250ml round-bottomed flask, add 200ml of a mixed solution of concentrated hydrochloric acid and deionized water with a volume ratio of 1:1, reflux at 125°C for 12 hours, wash with deionized water until neutral, at 100°C The activated mesoporous silica was obtained by vacuum drying for 24 hours. 3.02g of activated mesoporous silica gel was weighed, placed in a 100ml three-neck flask, 25ml of deionized water and 25ml of ethanol were added, stirred, and 5.5ml of γ-(methacryloyloxy)propyltrimethoxysilane was added dropwise. The reaction was carried out at 50°C for 24 hours under nitrogen protection. After cooling, filter, wash with ethanol and deionized water respectively, and vacuum dry at room temperature for 24 hours to obtain derivatized mesoporous silica gel. Infrared characterization results see Figure 4 , it can be seen from the figure that the derivatiz...
Embodiment 3
[0032] Take the surface-imprinted mesoporous silica gel prepared in Example 2, and fill it in a stainless steel column tube (50mm×4.6mm, I.D.) by homogenization, and the packing pressure is 35MPa, and use this self-made column to carry out the separation and purification of Cycloviral Buxus D. test (see Figure 5 ). The purified samples were tested to confirm that the purity increased from 65% to 98% (see Image 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com