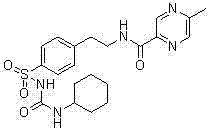
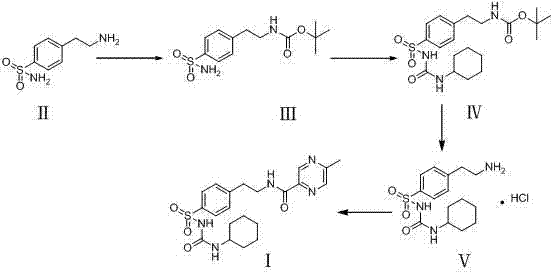
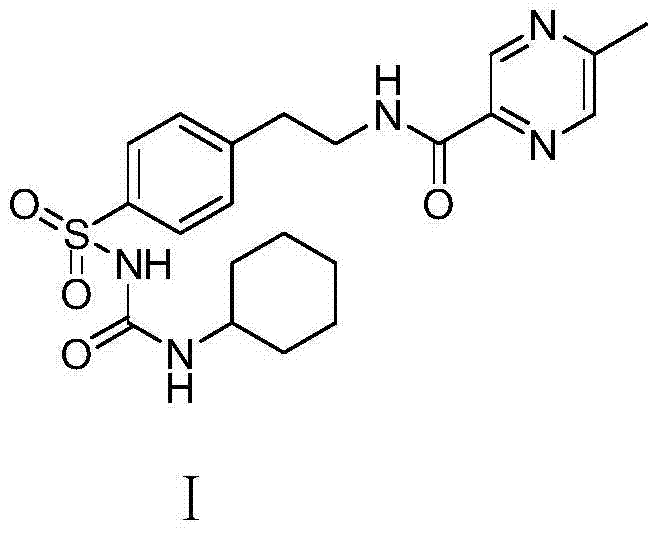
Novel synthesis route of glipizide
The technology of a glipizide and a synthesis method is applied in the preparation field of the hypoglycemic drug glipizide, can solve the problems of difficulty in obtaining starting materials, high cost, unfavorable industrialized production and the like, and achieves environmental friendliness, mild reaction conditions, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of compound III
[0027] Add 3.96 g (0.020 mol) 4-(2-aminoethyl) ammonium benzenesulfonate (compound II) into a 100 ml single-necked flask, and then add 75 ml of DMF and cool to 10°C under ice water bath and stir to dissolve. After dissolution, 4.8g Boc anhydride (0.022mol) was added dropwise to the reaction system at 0-5°C, and the temperature was raised to room temperature for 1.5-2h. After the reaction, the reaction solution was poured into 300 ml of purified water, a white solid was precipitated, and the compound III was dried to obtain 5.58 g of compound III solid with a yield of 93%.
Embodiment 2
[0028] Example 2: Preparation method 1 of compound IV
[0029] Add 5.54g (0.018mol) of compound III, 5.1g (0.037mol) of potassium carbonate, and 200ml of acetone into a 250ml three-necked flask, set up the condenser, stir and heat to reflux, and react for 6-7 hours. After the reaction, 2.78g (0.022mol) cyclohexyl isocyanate was added to the reaction system, and the reflux reaction was continued for 6-7h. After the reaction is completed again, suction filtration, the solid is taken out and transferred to a beaker, a small amount of purified water is added, and the pH is adjusted to 5-6 with 10% HCl solution, and then suction filtered, the solid is washed with purified water, and the compound IV solid is taken out and dried. 7.81g, the yield is 99.36%.
Embodiment 3
[0030] Example 3: Preparation method 2 of compound IV
[0031] Add 5.54g (0.018mol) of compound III, 3.9g (0.037mol) of sodium carbonate, and 200ml of acetone into a 250ml three-necked flask, set up the condenser, stir and heat to reflux, and react for 6-7 hours. After the reaction, 2.78g (0.022mol) cyclohexyl isocyanate was added to the reaction system, and the reflux reaction was continued for 6-7h. After the reaction is completed again, suction filtration, the solid is taken out and transferred to a beaker, a small amount of purified water is added, and the pH is adjusted to 5-6 with 10% HCl solution, and then suction filtered, the solid is washed with purified water, and the compound IV solid is taken out and dried. 7.81g, yield 99.36%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com