Method for quantitative detection of endogenous brassinosteroids in plant sample
A quantitative detection method, brassinosterol technology, applied in the field of analytical chemistry, can solve the problems of precious samples that cannot be realized, solvent consumption and high cost, etc., and achieve the effect of small sample size and effective detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
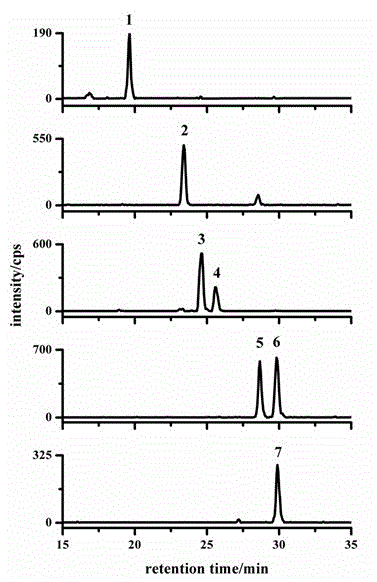
[0020] Determination of brassinosterols in rice leaves
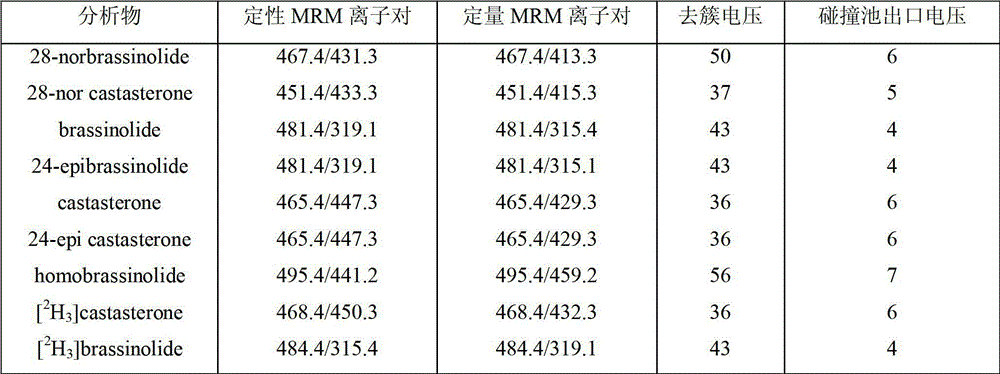
[0021] Accurately weigh 1g of rice leaf and place it in a mortar, freeze and grind it into powder with liquid nitrogen, transfer it to a 10mL centrifuge tube, and add the isotope internal standard [ 2 h 3 ] brassinolide and [ 2 h 3 ] Brassinosterone (castasterone) each 4ng, at the same time 5mL acetonitrile placed in the refrigerator at -18 ℃ for 12h extraction. The sample was centrifuged at 10,000 rpm for 10 min, and the supernatant was taken. Add 250 mg of sodium chloride to the supernatant, shake vigorously with a vortex mixer for 1 min to separate the aqueous phase and the organic phase, and discard the aqueous phase. Add 1 g of anhydrous magnesium sulfate to the centrifuge tube to further remove water. Centrifuge at 10,000 rpm for 10 min, and take the supernatant. Then the resulting supernatant was passed through a double-layer solid-phase extraction cartridge (previously activated by 5 mL of acetonitrile) thr...
Embodiment 2
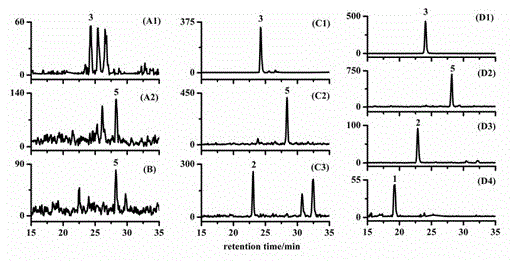
[0030] Determination of Brassinosterols in Rice Roots
[0031] Accurately weigh 1g of rice root and place it in a mortar, freeze and grind it into powder with liquid nitrogen, transfer it to a 10mL centrifuge tube, and add the isotope internal standard [ 2 h 3 ]brassinolide and [ 2 h 3 ] each 4ng of castasterone, and extract with 5mL of acetonitrile in a refrigerator at -20°C for 12h. The sample was centrifuged at 10,000 rpm for 10 min, and the supernatant was taken. Add 250 mg of sodium chloride to the supernatant, shake vigorously with a vortex mixer for 1 min to separate the aqueous phase and the organic phase, and discard the aqueous phase. Add 1 g of anhydrous magnesium sulfate to the centrifuge tube to further remove water. Centrifuge at 10,000 rpm for 10 min, and take the supernatant. Then the resulting supernatant was passed through a double-layer solid-phase extraction cartridge (previously activated by 5 mL of acetonitrile) through the solid-phase extraction ca...
Embodiment 3
[0033] Determination of Brassinosterols in Rice Flower Spikes
[0034] Accurately weigh 1g of rice flower spikes and place them in a mortar, freeze and grind them in liquid nitrogen until they are powdery, transfer them to a 10mL centrifuge tube, and add an isotope internal standard [ 2 h 3 ]brassinolide and [2 h 3 ] each 4ng of castasterone, and extract with 5mL of acetonitrile in a refrigerator at -20°C for 12h. The sample was centrifuged at 10,000 rpm for 10 min, and the supernatant was taken. Add 250 mg of sodium chloride to the supernatant, shake vigorously with a vortex mixer for 1 min to separate the aqueous phase and the organic phase, and discard the aqueous phase. Add 1 g of anhydrous magnesium sulfate to the centrifuge tube to further remove water. Centrifuge at 10,000 rpm for 10 min, and take the supernatant. Then the resulting supernatant was passed through a double-layer solid-phase extraction cartridge (previously activated by 5 mL of acetonitrile) through ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com