Preparation method of roflumilast
A technology of roflumilast and refined products, which is applied in the field of new special preparations, can solve problems such as high energy consumption, instability and easy hydrolysis of acyl chlorides, complicated operation steps, etc., and achieve simplified reaction steps, avoiding corrosion, and good product yield. The effect of rate and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
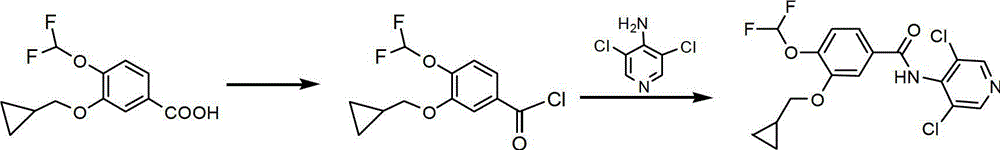
[0043] Embodiment 1 The preparation method of Roflumilast of the present invention
[0044]
[0045] (1) Add 47.4g of compound IV (4-amino-3,5-dichloropyridine) and 250ml of dry dichloromethane (DCM) into the reaction flask, stir to dissolve. Add 80g of N,N'-dicyclohexylcarbodiimide (DCC) in batches, and stir at room temperature for 40min. Then, 50 g of compound II (3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid) was dissolved in 250 ml of DCM, and the solution was added dropwise into the reaction flask, and the reaction was stirred at room temperature for 5 h after the addition.
[0046] (2) Filter out the white solid DCU (reaction by-product of DCC), then adjust the reaction solution to PH2~3 with 2mol / L HCl, filter out the solid, leave the solution to separate layers, and extract the aqueous layer with ethyl acetate for 2 Times (2*150ml). The combined organic layers were sequentially washed with saturated NaHCO 3Wash twice (2*150ml), wash twice with saturated NaCl...
Embodiment 2
[0050] Embodiment 2 The preparation method of Roflumilast of the present invention
[0051] (1) Add 4.74g of compound IV (4-amino-3,5-dichloropyridine) and 30ml of dry N,N-dimethylformamide (DMF) into the reaction flask, stir to dissolve. Add 8.0g of N,N'-dicyclohexylcarbodiimide (DCC) in batches, and stir at room temperature for 40min. Then, 5.0 g of compound II (3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid) dissolved in 30 ml of dry DMF was added dropwise to the reaction flask, and the reaction was stirred at room temperature for 5 h after the addition.
[0052] (2) Use 2mol / L HCl to adjust the reaction solution to PH2~3, filter out the solids, evaporate most of the DMF under reduced pressure, add 50ml of dichloromethane, stir for 30min and then a small amount of DCU solid precipitates, filter, and remove the dichloromethane from the mother liquor Add 30ml H after methane 2 O and 50ml of ethyl acetate, the solution was left to stand and the layers were separated, and...
Embodiment 3
[0053] Embodiment 3 The preparation method of Roflumilast of the present invention
[0054] (1) Add 4.74g of compound IV (4-amino-3,5-dichloropyridine) and 30ml of dry DMF into the reaction flask, stir to dissolve. Add 8.0g of N,N'-dicyclohexylcarbodiimide (DCC) in batches, and stir at room temperature for 40min. Then, 5.0 g of compound II (3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid) dissolved in 30 ml of dry DMF was added dropwise to the reaction flask, and the reaction was stirred at room temperature for 5 h after the addition.
[0055] (2) Adjust the reaction solution to pH 2~3 with 2mol / L HCl, filter off the solid, distill off most of the DMF under reduced pressure, add 30ml of HCl 2 O and 50ml of ethyl acetate, the solution was left to stand and the layers were separated, and the aqueous layer was extracted twice with ethyl acetate (2*30ml). The combined organic layers were sequentially washed with saturated NaHCO 3 Wash 2 times (2*30ml), wash 2 times with satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com