Preparation method of N-formamide compound
A technology for formamides and compounds, which is applied in the field of preparation of N-formamides, can solve the problems of troublesome post-processing, complicated operation and high cost, and achieve the effects of convenient post-processing, low risk and less generation of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
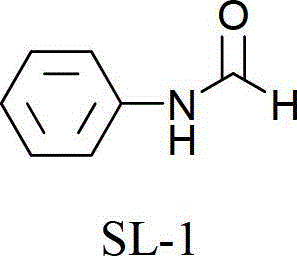
[0026] Embodiment 1: N-benzamide (SL-1)
[0027]
[0028] Add 3.93g (0.025mol) bromobenzene, 85mL (2.5mol) formamide, 0.20g (0.0025mol) copper oxide, 0.42g (0.0025mol) potassium iodide and 3.45g (0.025mol) potassium carbonate in a 250mL three-necked flask. Heat the reaction in an oil bath at 180°C for 3h, and stir mechanically. After the reaction is complete, the reaction liquid is cooled to room temperature, and after adding excess water, it is extracted with dichloromethane. After combining the organic phases, it is extracted once with saturated saline. After the organic layer is dried with anhydrous sodium sulfate and allowed to stand for a while, it is filtered. The filtrate was distilled under reduced pressure to obtain 2.71 g of the product with a yield of 89.5%.
[0029] 1 H-NMR (400MHz, CDCl 3 ):7.08-7.19(m,2H,Ar-H),7.32-7.38(m,2H,Ar-H),7.54(d,J=8.0Hz,1H,Ar-H),8.38(s,1H, CHO).
[0030] 13 C-NMR (100MHz, CDCl 3 ):δ=120.1(2C),125.2,129.7(2C),137.6,159.6.
[00...
Embodiment 5
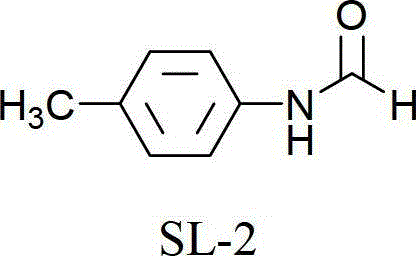
[0037] Embodiment 5: N-p-methylbenzamide (SL-2)
[0038]
[0039] The reaction steps are the same as in Example 1, except that the raw material is 0.025 mol of p-methylbromobenzene, which is heated and reacted in an oil bath at 120°C for 10 h to obtain 3.08 g of N-p-toluamide, with a yield of 91.2%.
[0040] 1 H-NMR (400MHz, CDCl 3 ):2.21(s,1H,CH 3 ), 7.20(d, J=8.0Hz, 2H, Ar-H), 7.54(d, J=8.0Hz, 2H, Ar-H), 8.70(s, 1H, CHO).
[0041] 13 C-NMR (100MHz, CDCl 3 ):δ=22.3, 120.8(2C), 132.8(2C), 135.4, 137.6, 160.2.
Embodiment 6
[0042] Embodiment 6: N-butyl formamide (SL-3)
[0043]
[0044] Add 3.42g (0.025mol) of 1-bromobutane, 0.96mL (0.025mol) of formamide, 20mL of toluene, 0.42g (0.0025mol) of potassium iodide and 2.5g (0.025mol) of potassium bicarbonate into a 100mL three-necked flask. Heat at 120°C under reflux in an oil bath for 6 hours, and stir mechanically. After the reaction is complete, cool the reaction solution to room temperature, remove part of the solvent by rotary evaporation, add excess water to the residue and extract it with chloroform, combine the organic phases and extract it once with saturated saline, and dry the organic layer with anhydrous sodium sulfate After standing for a period of time, it was filtered, and the filtrate was distilled under reduced pressure to obtain 2.36 g of the product, with a yield of 93.4%.
[0045] 1 H-NMR (400MHz, CDCl 3 ):0.9(t,J=7.7Hz,3H,CH 3 ),1.28-1.36(m,2H,CH 2 ),1.48-1.54(m,2H,CH 2),3.14-1.20(m,2H,CH 2 ), 8.04(s,1H,CHO).
[0046] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com