Venlafaxine hydrochloride controlled-release pellet and preparation method thereof
A technology of venlafaxine hydrochloride and sustained-release pellets, applied in the field of medicine, can solve the problems of large side effects, short half-life, and patients need frequent administration, and achieves small influence of individual differences, stable blood drug concentration, and reduced gastrointestinal effect of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
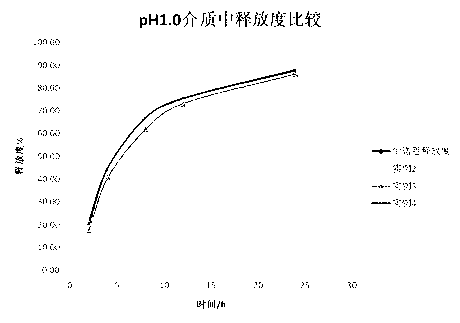
Image
Examples
Embodiment 1
[0026] The venlafaxine hydrochloride sustained-release pellets provided by the present invention are composed of three parts: a blank pellet core, a venlafaxine hydrochloride drug-containing layer evenly covering the surface of the blank pellet core, and a sustained-release pellet uniformly covering the surface of the drug-containing layer. membrane.
[0027] 1. Choice of blank pill heart
[0028] Blank cores can be selected from pharmaceutically acceptable cores such as sucrose pellets, microcrystalline cellulose pellets, starch pellets, and lactose pellets. Qualified samples can be prepared when the particle size distribution is between 500-1000 μm. The present invention has passed a large number of experiments. It is found that when the core of blank pellets is sucrose pellets, the particle size of 710-850μm is the optimal choice. Through the RSD comparison test of the release degree, it is proved that the particle size of 710-850μm is the best.
[0029] Table 1: Influenc...
Embodiment 2
[0038] The preparation process of the pills described in this example is as follows: 3.1 kg of blank ball cores with a particle size distribution of 710-850 μm are added to the fluidized bed, and 1.9 kg of venlafaxine hydrochloride and 190 g of talcum powder are suspended in 3.8 kg75 % ethanol solution, the suspension is evenly sprayed on the surface of the pellet core by the bottom spraying process. The main parameters of the drug in the fluidized bed are recorded as follows:
[0039] parameters
value
Inlet air temperature
55℃
material temperature
45℃
Liquid supply speed
50rpm
Fan frequency
25Hz
atomization pressure
0.14Mpa
[0040] An appropriate amount of talcum powder is added to the prescription, and 75% ethanol solution is used instead of distilled water as a solvent, which greatly reduces the viscosity of the solution, makes the drug application process continuous and fast, and the pellets have n...
Embodiment 3
[0045] The preparation of containing pill is the same as embodiment 2.
[0046] The preparation process of the slow-release pill described in the present embodiment is specifically as follows: 250g EC (45 centipoise), 12.5g polyacrylic resin III, 12.5g HPMC E5, 25g castor oil are dissolved in 6.25kg75% ethanol solution, and pass flow The coating solution was sprayed onto the surface of the 5 kg pill-containing pellets prepared in Example 1 by chemical bed bottom spraying process to prepare the sustained-release pellet preparation. The main parameters of fluidized bed coating are recorded as follows:
[0047] parameters
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com