Method for selecting 1-deoxy-D-xylulose-5-phosphate synthase inhibitor from plant extract
A plant extract and inhibitor technology, applied in the field of enzyme inhibitor screening, can solve problems such as large-scale and expensive, and achieve the effects of strong feasibility, high sensitivity and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] 2) Preparation of control samples
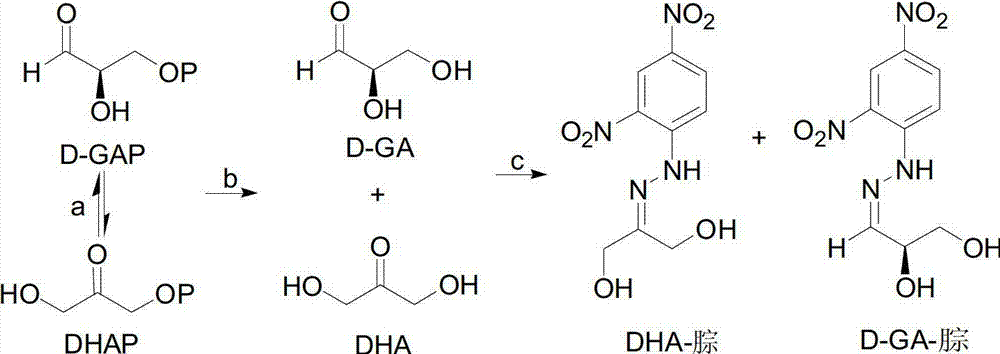
[0039] 2-10mM D-GAP (or DHAP), 5-20mM Mg 2+ or Zn 2+ , 0.2-2mg / ml of DXS enzyme is added to 40-400mM Tris-HCl or PBS (pH5.0-9.0) buffer solution, and the reaction system can prepare the control sample;
[0040] The difference between the sample prepared in step 1) for detecting DXS enzyme inhibitors and the control sample prepared in step 2) is that no plant extract is added to the control sample, and the other components are the same.
[0041] In actual experimental operation, the total volume of the reaction system in step 1) and step 2) is respectively 50-200 μ l (in the control sample of step 2), no plant extract is added, and the volume is replenished with ultrapure water), the volume of The selection can ensure that the reaction can be completely carried out under the condition of detection, and the substrate will not be wasted. The reaction temperature is 30-40°C, which ensures the activity of the enzyme. The reaction time is...
specific Embodiment 1
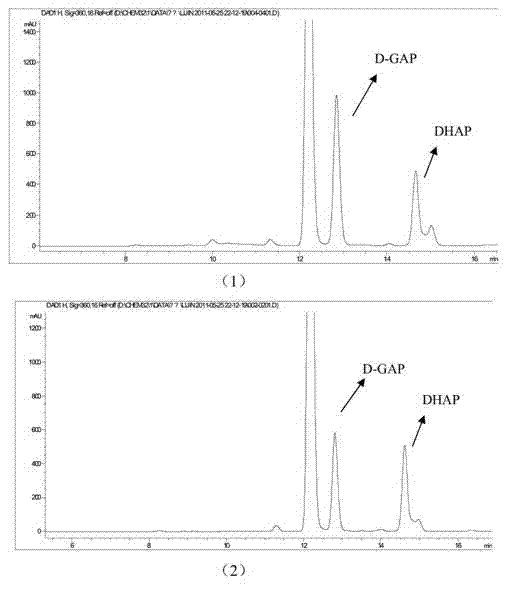
[0056] Specific Example 1: Using D-GAP as a substrate
[0057] 1. Isomerization reaction
[0058] 1) Preparation of samples for detection of DXS enzyme inhibitors:
[0059] 5mM D-GAP, 20mM Mg 2+ , 0.5mg / ml of DXS enzyme was added to 60mM Tris-HCl (pH8.0) buffer, and then 20μg / μl of plant extract was added, and the reaction system was reacted in a water bath at 37°C for 2h to prepare a Samples of DXS enzyme inhibitors;
[0060] 2) Preparation of control samples
[0061] A control sample was prepared in the same manner as above, and the reactants and concentrations in the control sample were the same as in 1), the difference being that no plant extract was added to the control sample, and the volume was supplemented with ultrapure water.
[0062] 2. Enzyme digestion reaction
[0063] After the isomerization reaction, take 50 μl of the reaction product in 1, add 6 μl of 10×CIAP buffer, add 1 unit of alkaline phosphatase, and incubate at 37°C for 2 hours.
[0064] 3. Derivat...
specific Embodiment 2
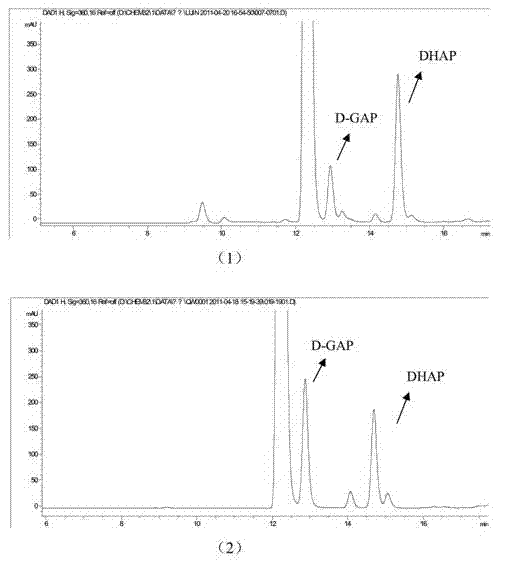
[0072] Specific embodiment two: take DHAP as substrate
[0073] 1. Isomerization reaction
[0074] 1) Preparation of samples for detection of DXS enzyme inhibitors:
[0075] 5mM DHAP, 20mM Mg 2+ , 0.5mg / ml of DXS enzyme was added to 60mM Tris-HCl (pH8.0) buffer, and then 20μg / μl of plant extract was added, and the reaction system was reacted in a water bath at 37°C for 2h to prepare a Samples of DXS enzyme inhibitors;
[0076] 2) Preparation of control samples
[0077] A control sample was prepared in the same manner as above, and the reactants and concentrations in the control sample were the same as in 1), the difference being that no plant extract was added to the control sample, and the volume was supplemented with ultrapure water.
[0078] 2. Enzyme digestion reaction
[0079] After the isomerization reaction, take 50 μl of the reaction product in 1, add 6 μl of 10×CIAP buffer, add 1 unit of alkaline phosphatase, and incubate at 37°C for 2 hours.
[0080] 3. Derivat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com