Fingerprint constructing method of total flavonoid components and total alkaloids components in loranthus parasiticus-kudzuvine root preparation and quality detecting method
A technology of fingerprint spectrum and total alkaloids, which is applied in the field of quality inspection of mulberry preparations, can solve the problems that the quality of mulberry preparations cannot be controlled, cannot fully reflect the content of total flavonoids and total alkaloids active ingredients, etc. Safety, good reproducibility, and the effect of protecting rights and interests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
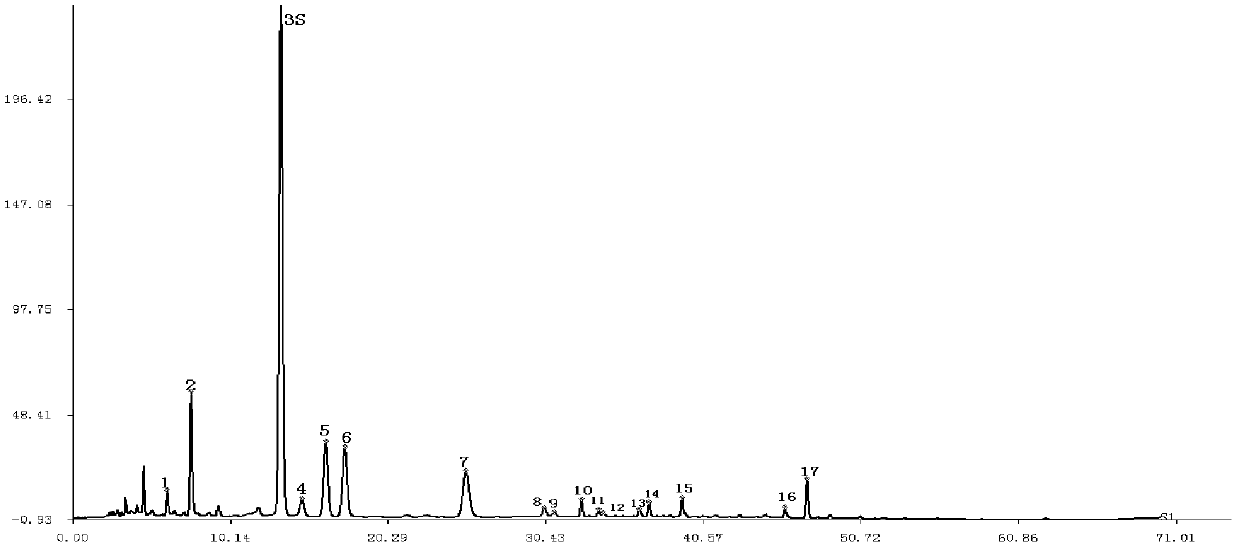
[0089] Example 1 Construction of Sangge Preparation Total Flavonoids Control Fingerprint
[0090] The 10 batches of Morus alba medicinal materials and Pueraria radiata used in Example 1 are mainly produced in Henan, Hebei, Anhui, Guangxi, and Hunan. After identification, the above-mentioned varieties meet the pharmacopoeia standards and have no obvious biological differences. For details, please refer to Table 1:
[0091] Table 1
[0092]
[0093] Morus alba
[0094] Table 2 shows the specific batch numbers of Cortex Mori and Radix Puerariae in 10 batches of Sangge preparations.
[0095] Table 2
[0096] Sangge preparation number
[0097] Instruments used: SHIMADZU HPLC including LC-20AT chromatographic pump, SPD-M20A UV detector, CBM-20A controller, DGU-20A5 degasser, SIL-20A autosampler, CTO-10AS column thermostat , class-vp workstation; chromatographic column: Apollo-C18 (250×4.6mm, 5μm) analytical column.
[0098] Preparation of reference s...
Embodiment 2
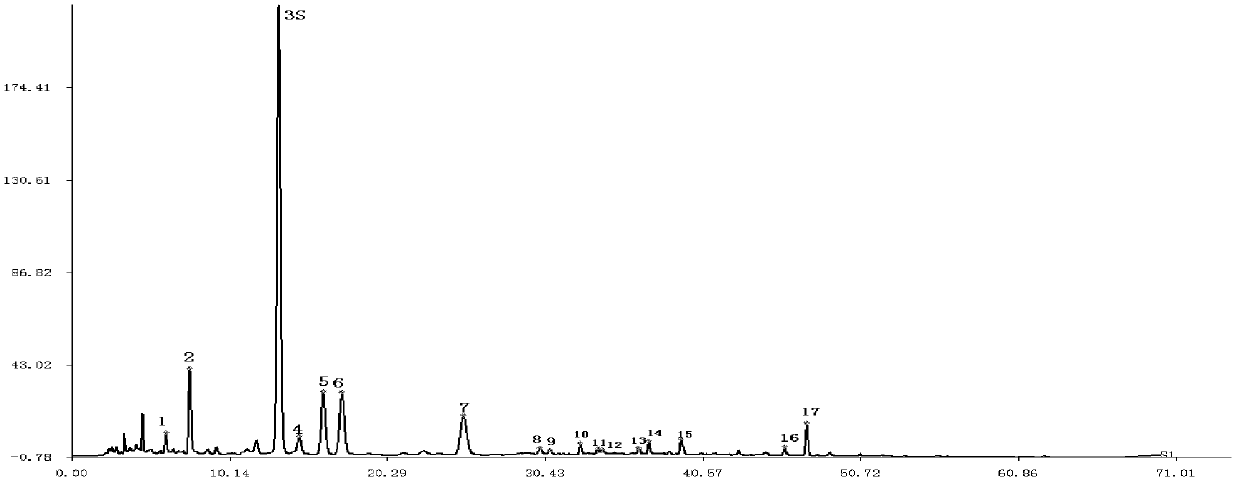
[0124] Embodiment 2 is to the quality detection method of total flavonoids composition in the Sangge preparation
[0125] The source of the Sangge preparation to be tested:
[0126] From Morus alba extract S20100721 (extracted from Morus alba medicinal material with batch number Y070609HN) and kudzu root extract G20100813 (extracted from kudzu root medicinal material with batch number G110118HN) according to the ratio of medicinal materials 2:1, 4:1, 1 :2, 1:4 mixed.
[0127] The preparation and chromatographic conditions of the instrument used, the reference substance solution, the Sangge preparation solution to be tested are all the same as in Example 1.
[0128] Precisely draw 10 μl each of the reference substance solution and the Sangge preparation solution to be tested, inject into the liquid chromatograph, measure, and record the chromatogram for 70 minutes. For the fingerprints of the total flavonoid components of the Sangge preparation to be tested, please see the ...
Embodiment 3
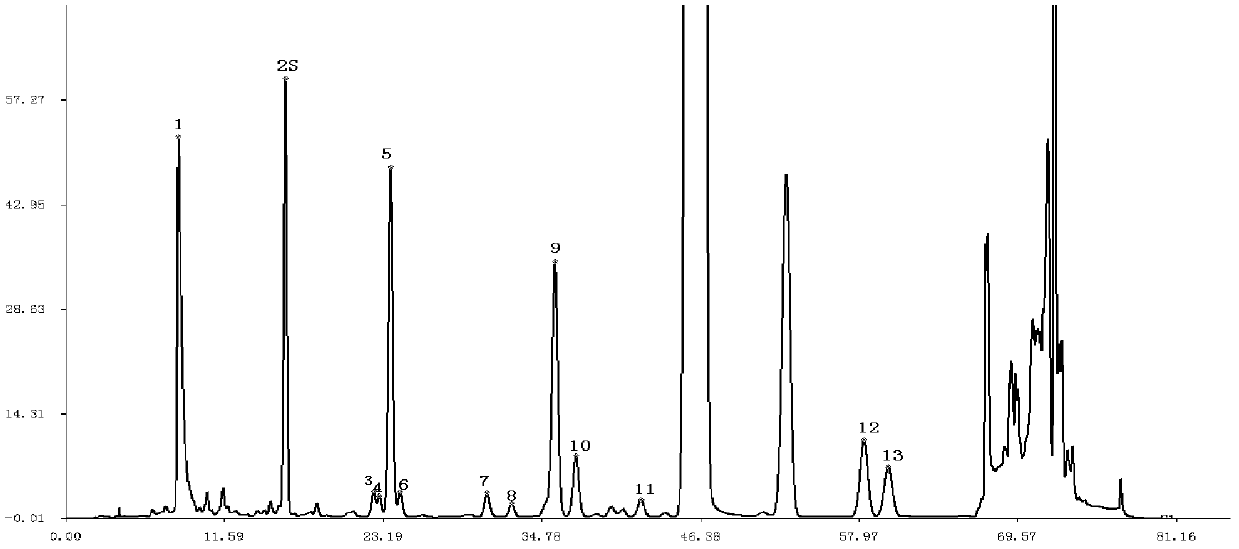
[0130] Example 3 Construction of fingerprints of total alkaloid components in Sangge preparations
[0131] Instruments used: SHIMADZU HPLC including LC-20AT chromatographic pump, RF-20A fluorescence detector, CBM-20A controller, DGU-20A5 degasser, SIL-20A autosampler, CTO-10AS column thermostat , class-vp workstation; chromatographic column: CosmosilC18 (250×4.6mm, 5μm) analytical column
[0132] Preparation of reference solution: Accurately weigh an appropriate amount of 1-deoxynojirimycin hydrochloride reference substance (Sigma Company, batch number 078K4062), add water to make a solution containing 4 μg per 1 ml;
[0133] 1) Preparation of Sangge preparation solution: each batch of Sangge preparations was prepared according to the mass ratio of Morus alba and Pueraria 2:1, 4:1, 1:2, and 1:4 to prepare four Sangge preparations . Take by weighing about 0.05g of each of the above Sangge preparations, accurately weigh, put in a 100ml conical flask with a stopper, accurately ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com