Composition containing pravastatin calcium as well as preparation method and application thereof
A technology of pitavastatin calcium and a composition is applied in the field of a composition containing pitavastatin calcium and its preparation field, which can solve the problems of rareness, rational combination of pitavastatin calcium and health-care drugs and no relevant reports, etc. therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
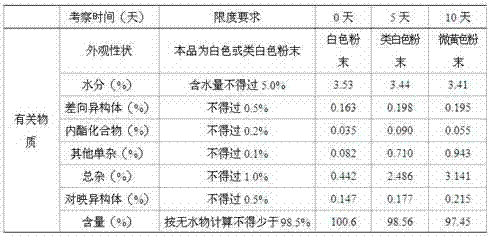
[0028] A coated tablet composed of pitavastatin calcium and amino acid comprises a tablet core and a coating. Wherein the components of the tablet core include by weight parts: pitavastatin calcium 10g, L-arginine 2057g, taurine 614g, L-citrulline 527g, vitamin E 112g, lactose 712g, microcrystalline cellulose 518g, Low-substituted hydroxypropyl cellulose 323g, crospovidone 80g, 8% povidone-K30 95% ethanol solution 350g (povidone-K30 is dissolved in the solution that forms in 95%w / w ethanol, its weight hundred content of 8%), micronized silica gel 19.5g, magnesium stearate 21.2g, Opadry Y-1-7000 150.1g.
[0029] Implementation steps:
[0030] (1) The prescribed amount of pitavastatin calcium, L-arginine, taurine, L-citrulline, vitamin E, lactose, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and cross-linked polyvinyl chloride Ketone, micropowder silica gel, and magnesium stearate are dried at 60°C, and the moisture content is ≤2.0% by weight;
[0031]...
Embodiment 2
[0039] A coated tablet composed of pitavastatin calcium and amino acid comprises a tablet core and a coating. Wherein each component of the tablet core includes by weight: (prepared each coated tablet containing pitavastatin calcium 1mg)
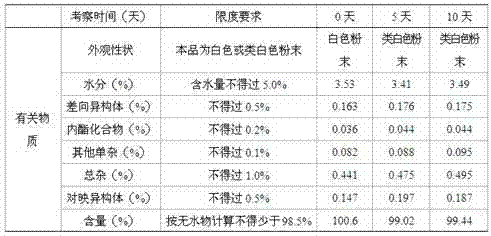
[0040] Pitavastatin calcium 10g, L-arginine 2532g, taurine 716g, L-citrulline 705g, vitamin E 154g, lactose 821g, microcrystalline cellulose 704g, low-substituted hydroxypropyl cellulose 353g, cross-linked polymer Povidone 80g, 8% povidone-K30 95% ethanol solution 350g (Povidone-K30 is dissolved in 95%w / w ethanol to form a solution, its weight percentage is 8%), micropowder silica gel 25g, hard Magnesium fatty acid 25g, Opadry Y-1-7000 185.4g.
[0041] Implementation steps: with embodiment 1.
Embodiment 3
[0043] A coated tablet composed of pitavastatin calcium and amino acid comprises a tablet core and a coating. Wherein each component of the tablet core includes by weight: (prepared each coated tablet containing pitavastatin calcium 1mg)
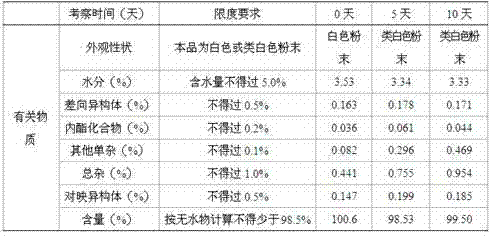
[0044] Pitavastatin calcium 10g, L-arginine 1800g, taurine 530g, L-citrulline 450g, vitamin E 86g, lactose 508g, microcrystalline cellulose 527g, low-substituted hydroxypropyl cellulose 292g, cross-linked polymer Povidone 80g, 8% povidone-K30 95% ethanol solution 350g (Povidone-K30 is dissolved in 95%w / w ethanol to form a solution, its weight percentage is 8%), micropowder silica gel 23g, hard Magnesium fatty acid 18g, Opadry Y-1-7000 128g
[0045] Implementation steps: with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com