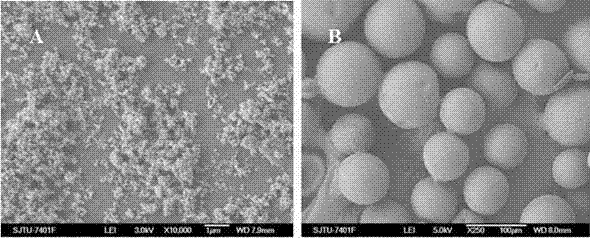
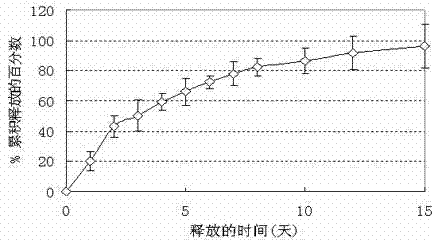
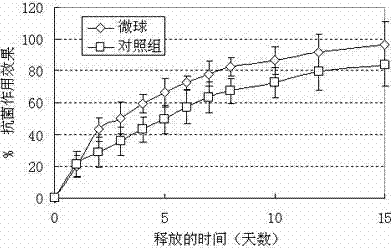
Adriamycin-containing nanometer medicament microspheres and preparation method thereof
A nano-drug, doxorubicin technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problem of low encapsulation rate, incomplete release and burst release, local microencapsulation and inflammation To achieve the effect of enhancing cell adhesion, reducing inflammation and microencapsulation, regular particles without adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of polylactic-glycolic acid (PLGA) microspheres loaded with doxorubicin nano-medicine comprises the following steps:
[0051] (1) Dissolve 20 mg of doxorubicin in 0.5 ml of water, then stir with 20 mg of porous silica nanoparticles for 24 hours, so that the doxorubicin is fully adsorbed in the porous silica nanoparticles, and centrifuge to remove the supernatant solution, fully washed 3 times, and then freeze-dried to form adriamycin nano-medicine;
[0052] (2) Mix the above-mentioned doxorubicin nanomedicine with 20% (w / w) PLGA in dichloromethane solution at a weight ratio of 1:9 and sonicate for 5 minutes to form a uniform suspension, that is, doxorubicin in oil Suspension of element nano drug (N / O);
[0053] (3) Add the doxorubicin in oil nano drug (N / O) suspension obtained in step (2) dropwise into 50 ml of silver nanoparticle aqueous suspension with a concentration of 10% (w / w), and stir for 5 Form nanoparticle suspension oil-in-oil-in-oil doxorub...
Embodiment 2
[0061] The preparation of polycaprolactone (PCL) microspheres loaded with doxorubicin nanomedicine comprises the following steps:
[0062] (1) Dissolve 20 mg of doxorubicin in 0.5 ml of water, then stir with 20 mg of porous titanium dioxide nanoparticles for 24 hours, so that the doxorubicin is fully adsorbed in the porous titanium dioxide nanoparticles, centrifuge to remove the supernatant, and then fully Washed 3 times, then freeze-dried to form doxorubicin nanomedicine;
[0063] (2) Mix the above-mentioned doxorubicin nanomedicine with the ethyl acetate solution of PCL with a concentration of 0.5% (w / w) according to the weight ratio of 1:10 and ultrasonicate for 5 minutes to form a uniform suspension, that is, doxorubicin in oil Suspension of element nano drug (N / O);
[0064] (3) Add the doxorubicin in oil nano-drug (N / O) suspension obtained in step (2) dropwise to 50 ml containing 10% (w / w) silver nanoparticles and 1% (w / w) polyethylene In the aqueous suspension of alcoh...
Embodiment 3
[0070] The preparation of polylactic acid (PLA) microspheres loaded with doxorubicin nano-medicine comprises the following steps:
[0071] (1) Dissolve 10 mg doxorubicin and 10 mg dextran into 0.4 ml of water to form a drug aqueous solution, and then transfer the above solution to 3.2 ml of polyethylene glycol (PEG8000) with a concentration of 5% (w / w) Mix well in aqueous solution, then pre-freeze in -80°C refrigerator for 12 hours, then freeze-dry with a freeze dryer, then dissolve PEG with dichloromethane and centrifuge to remove PEG to obtain adriamycin nano-medicine;
[0072] (2) Mix the above-mentioned doxorubicin nanomedicine with PLA acetonitrile solution with a concentration of 30% (w / w) at a weight ratio of 1:8, and ultrasonicate for 1 minute to form a uniform suspension, that is, doxorubicin in oil Suspension of element nano drug (N / O);
[0073] (3) Add the doxorubicin in oil nano drug (N / O) suspension obtained in step (2) dropwise into 40 ml of 20% (w / w) hydroxyapa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com