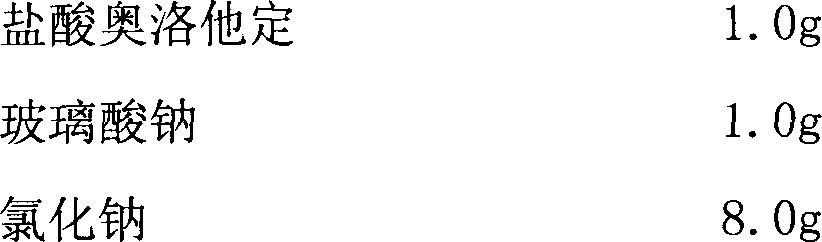Novel olopatadine hydrochloride eye drop and preparation method thereof
A technology of olopatadine hydrochloride and eye drops, applied in the field of medicine, can solve problems such as no reports of olopatadine hydrochloride eye drops and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Preparation method:
[0022] Dissolve olopatadine hydrochloride and related auxiliary materials in appropriate amount of water for injection respectively.
[0023] Take the prescribed amount of sodium hyaluronate in water for injection, stir continuously at 60-70°C to make it fully swell, boil, and filter while hot with a microporous membrane.
[0024] Slowly add the filtered sodium hyaluronate solution into the olopatadine hydrochloride solution, stir evenly while adding, add water for injection to a sufficient amount, filter to obtain a clarified solution, sub-package, quality test, and only if it is qualified.
Embodiment 2
[0026] prescription:
[0027]
[0028]
[0029] Preparation method:
[0030] Take olopatadine hydrochloride, sodium chloride and related auxiliary materials and dissolve them in appropriate amount of water for injection respectively.
[0031] Take the prescribed amount of sodium hyaluronate in water for injection, stir continuously at 60-70°C to make it fully swell, boil, and filter while hot with a microporous membrane.
[0032] Slowly add the filtered sodium hyaluronate solution into the olopatadine hydrochloride solution, stir evenly while adding, add water for injection to a sufficient amount, filter to obtain a clarified solution, sub-package, quality test, and only if it is qualified.
Embodiment 3
[0034] prescription:
[0035]
[0036] Preparation method:
[0037] Take olopatadine hydrochloride, sodium chloride and related auxiliary materials and dissolve them in appropriate amount of water for injection respectively.
[0038] Take the prescribed amount of sodium hyaluronate in water for injection, stir continuously at 60-70°C to make it fully swell, boil, and filter while hot with a microporous membrane.
[0039] Slowly add the filtered sodium hyaluronate solution into the olopatadine hydrochloride solution, stir evenly while adding, add water for injection to a sufficient amount, filter to obtain a clarified solution, sub-package, quality test, and only if it is qualified.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com