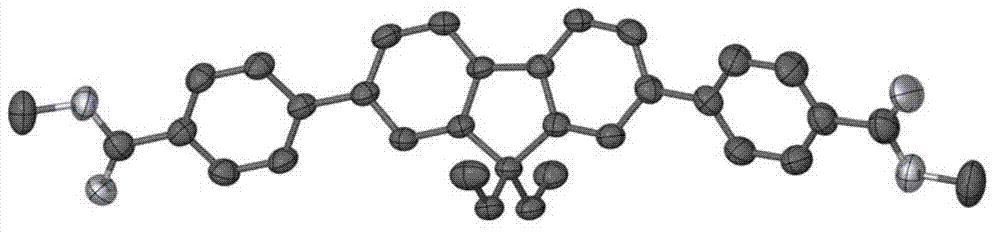
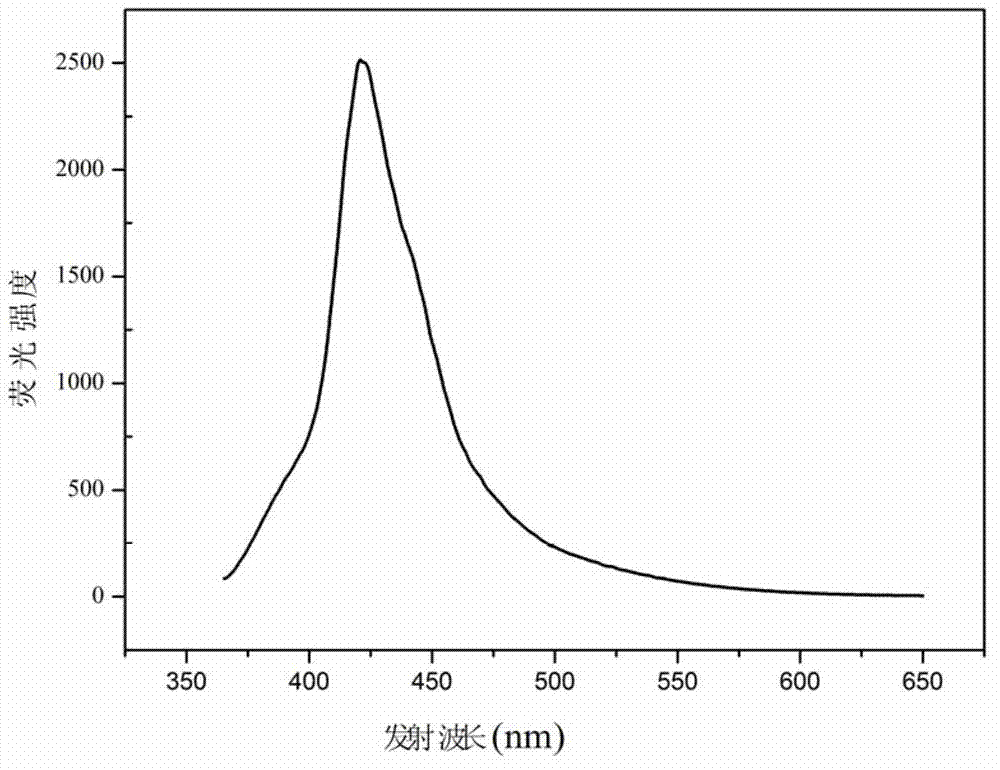
Fluorene acid ester fluorescent material and preparation method thereof
A technology of fluorene carboxylate and fluorescent material, which is applied in the field of fluorene carboxylate fluorescent material and its preparation, can solve problems such as harsh preparation conditions, and achieve the effects of less harsh synthesis conditions, enhanced luminescence performance, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Take solid powder material 9,9-diethyl-2,7-dibromofluorene (1.29g, 4mmol), 4-methoxycarbonylphenylboronic acid (1.62g, 9mmol), tetrakistriphenylphosphine palladium (0.300g, 0.26mmol), potassium carbonate (2.07g, 15mmol) were mixed in a 250mL three-necked flask; the three-necked flask was evacuated and filled with nitrogen three times; The mixed solution was reacted at 80°C for 2 days under the protection of nitrogen, and the reaction process was tracked by thin-layer chromatography during the reaction; after the reaction was completed and the reaction solution was cooled, the aqueous phase was extracted with dichloromethane, and then washed with saturated brine to remove impurities , then dried with anhydrous magnesium sulfate, filtered; spin-dried toluene, ethanol and dichloromethane in the filtrate to obtain a solid mixture, the mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 15: 1 was used as an eluent , separated and purified by column chro...
Embodiment 2
[0022] Take solid powder material 9,9-diethyl-2,7-dibromofluorene (1.29g, 4mmol), 4-methoxycarbonylphenylboronic acid (2.16g, 12mmol), tetrakistriphenylphosphine palladium (0.185g, 0.16mmol), potassium carbonate (2.77g, 20mmol) were mixed in a 250mL three-necked flask; the three-necked flask was evacuated and filled with nitrogen 4 times; then a mixture of 60mL of toluene, 25mL of ethanol and 15mL of water was injected for 20min in addition to peroxygen Liquid, reacted at 85°C for 2 days under the protection of nitrogen, and tracked the reaction process with thin-layer chromatography during the reaction; after the reaction was completed and the reaction liquid was cooled, the aqueous phase was extracted with dichloromethane, and then washed with saturated brine to remove impurities. Dry with anhydrous magnesium sulfate afterwards, filter; Spin-dry the toluene in the filtrate, ethanol and methylene dichloride to obtain solid mixture after, with the mixed solvent that sherwood oi...
Embodiment 3
[0024] Take solid powder material 9,9-diethyl-2,7-dibromofluorene (1.61g, 5mmol), 4-methoxycarbonylphenylboronic acid (3.24g, 18mmol), tetrakistriphenylphosphine palladium (0.462g, 0.40mmol), potassium carbonate (4.15g, 30mmol) were mixed in a 250mL three-necked flask; the three-necked flask was evacuated and filled with nitrogen for 3 times; then a mixture of toluene 90mL, ethanol 50mL and water 30mL was injected for 25min in addition to peroxygen liquid, reacted at 95°C for 3 days under the protection of nitrogen, and tracked the reaction process with thin-layer chromatography during the reaction; after the reaction was completed and the reaction liquid was cooled, the aqueous phase was extracted with dichloromethane, and then washed with saturated brine to remove impurities. Dry with anhydrous magnesium sulfate afterwards, filter; Spin-dry the toluene in the filtrate, ethanol and methylene dichloride to obtain solid mixture after, with the mixed solvent that sherwood oil and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com