Anti-inflammatory analgesia mutual prodrug of non-steroidal antiinflammatory drug and preparation method thereof
A non-steroidal anti-inflammatory and prodrug technology, applied in the direction of anti-inflammatory agents, carboxylic acid amide preparation, non-central analgesics, etc., can solve the restrictions on the wide use of NSAID drugs, the inability to take oral administration, and the instability of the gastrointestinal system and other problems, to achieve good biological activity, high safety, and prolonged effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Synthesis of synergistic prodrug paracetamol-flurbiprofen (AF)
[0030] Add 2g flurbiprofen (8.2mmol) in a 25ml single-necked round bottom flask, then add 15ml SOCl dropwise 2 , heated and stirred to reflux for 4-5 hours, and TLC detected that the conversion of flurbiprofen was complete and the reaction was stopped. Remove excess SOCl by distillation under reduced pressure 2 Finally, the flurbiprofen after acid chloride was transferred to a 50ml single-necked round bottom flask and dissolved in 20ml CH 2 Cl 21.24 g of acetaminophen (8.2 mmol) was dissolved in CH 2 Cl 2 in spare. Slowly add 8.2 mmol of acetaminophen in CH with a constant pressure dropping funnel 2 Cl 2 solution, remove the ice-water bath after the dropwise addition, stir and react at room temperature for 3 hours, and stop the reaction after the complete conversion of paracetamol as detected by TLC. The reaction solution is directly precipitated with ice water and filtered to obtain ...
Embodiment 2
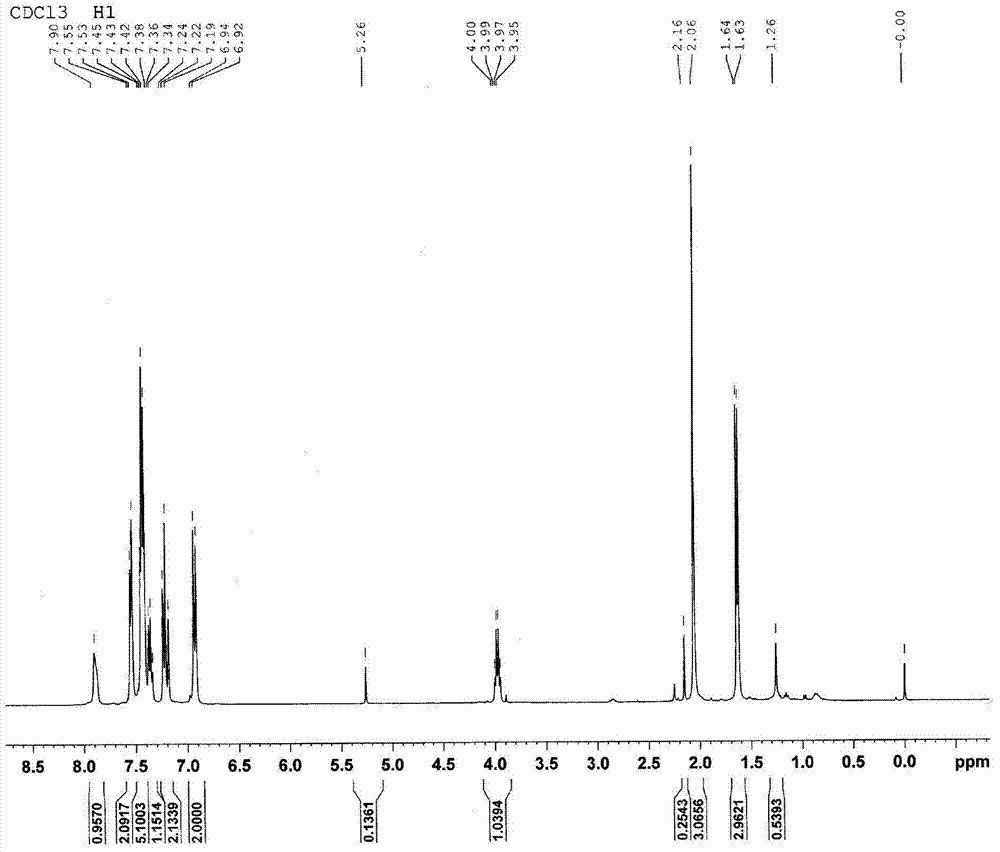
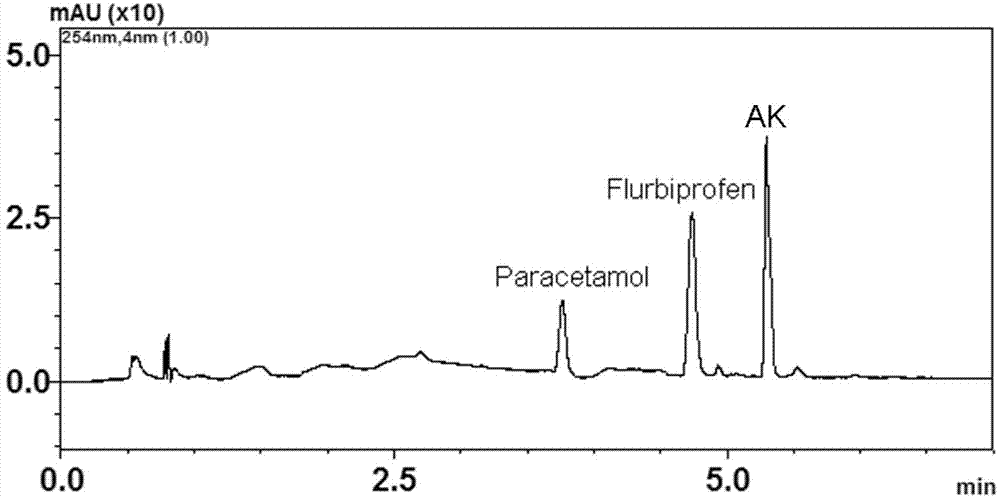
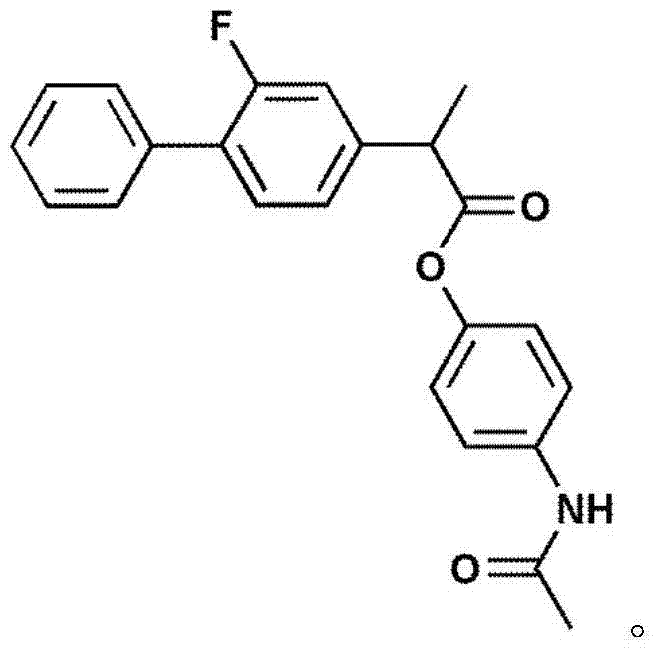
[0032] Embodiment 2: the synthesis of synergistic prodrug paracetamol-ketoprofen (AK)
[0033] In a 50ml single-necked round bottom flask, add 2g of ketoprofen (8.0mmol) acid chloride and dissolve in 20ml CH 2 Cl 2 1.21 g of acetaminophen (8.0 mmol) was dissolved in CH 2 Cl 2 in spare. Slowly add 8.2 mmol of acetaminophen in CH with a constant pressure dropping funnel 2 Cl 2 solution, remove the ice-water bath after the dropwise addition, stir and react at room temperature for 3 hours, and stop the reaction after the complete conversion of paracetamol as detected by TLC. The reaction solution is directly precipitated with ice water, and then filtered to obtain a crude product, which is further crystallized with acetone to obtain acetaminophen-ketoprofen prodrug with a purity ≥ 99.5%. The synthesis process is as follows:
[0034]
Embodiment 3
[0035] Embodiment 3: Synthesis of synergistic prodrug acetaminophen-pirprofen (AP)
[0036] In a 50ml single-necked round bottom flask, add 2g of chlorinated pyrprofen (8.0mmol) and dissolve it in 20ml of CH 2 Cl 2 , stirred in an ice-water bath and added 1ml of triethylamine as an acid-binding agent. Dissolve 1.2 g of acetaminophen (8.0 mmol) in CH 2 Cl 2 Slowly add 8.2 mmol of acetaminophen in CH with a constant pressure dropping funnel. 2 Cl 2 solution, remove the ice-water bath after the dropwise addition, stir and react at room temperature for 3 to 4 hours, and stop the reaction after TLC detects that the conversion of the raw materials is complete. Afterwards, the reaction solution was directly applied to flash column chromatography (normal phase silica gel chromatography, 300-400 mesh), and washed with dichloromethane and dichloromethane-methanol (volume ratio 9:1, 4:1, 1:1) respectively. Remove 2 column volumes, collect the eluate of each section and suspend evap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com