Preparation and application of recombinant Mycobacterium smegmatis expressing NY-ESO-1
A NY-ESO-1, Mycobacterium smegmatis technology, applied in the fields of biology and genetic engineering, can solve problems such as patient harm and tuberculosis infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0095] The preparation method of recombinant Mycobacterium smegmatis of the present invention may comprise the steps: (1) providing a plasmid that can be used to express the recombinant protein of the present invention; (2) transforming Mycobacterium smegmatis with said plasmid to obtain the recombinant Mycobacterium smegmatis of the present invention Mycobacteria; and (3) optionally culturing, expanding and / or isolating the obtained recombinant Mycobacterium smegmatis.
[0096] Transformation can be carried out by conventional transformation methods of the present invention such as electroporation and protoplast transformation. The fusion protein produced by the recombinant Mycobacterium smegmatis of the present invention can stimulate the immune system of mammals to produce antibodies against NY-ESO-1.
[0097] In a preferred embodiment of the present invention, a Mycobacterium smegmatis bacterial strain selected from the following group can be used to construct the recombin...
Embodiment 1
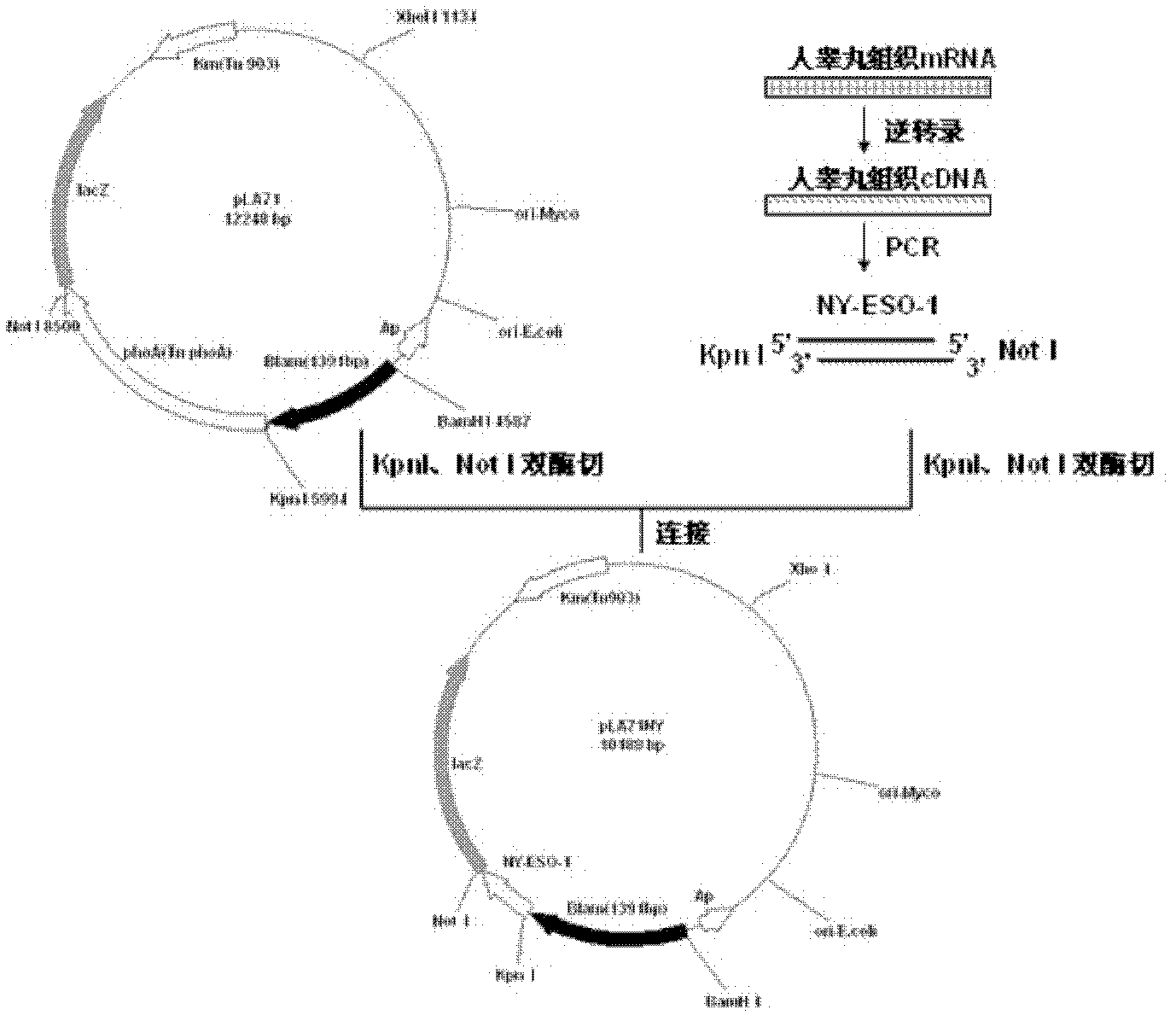
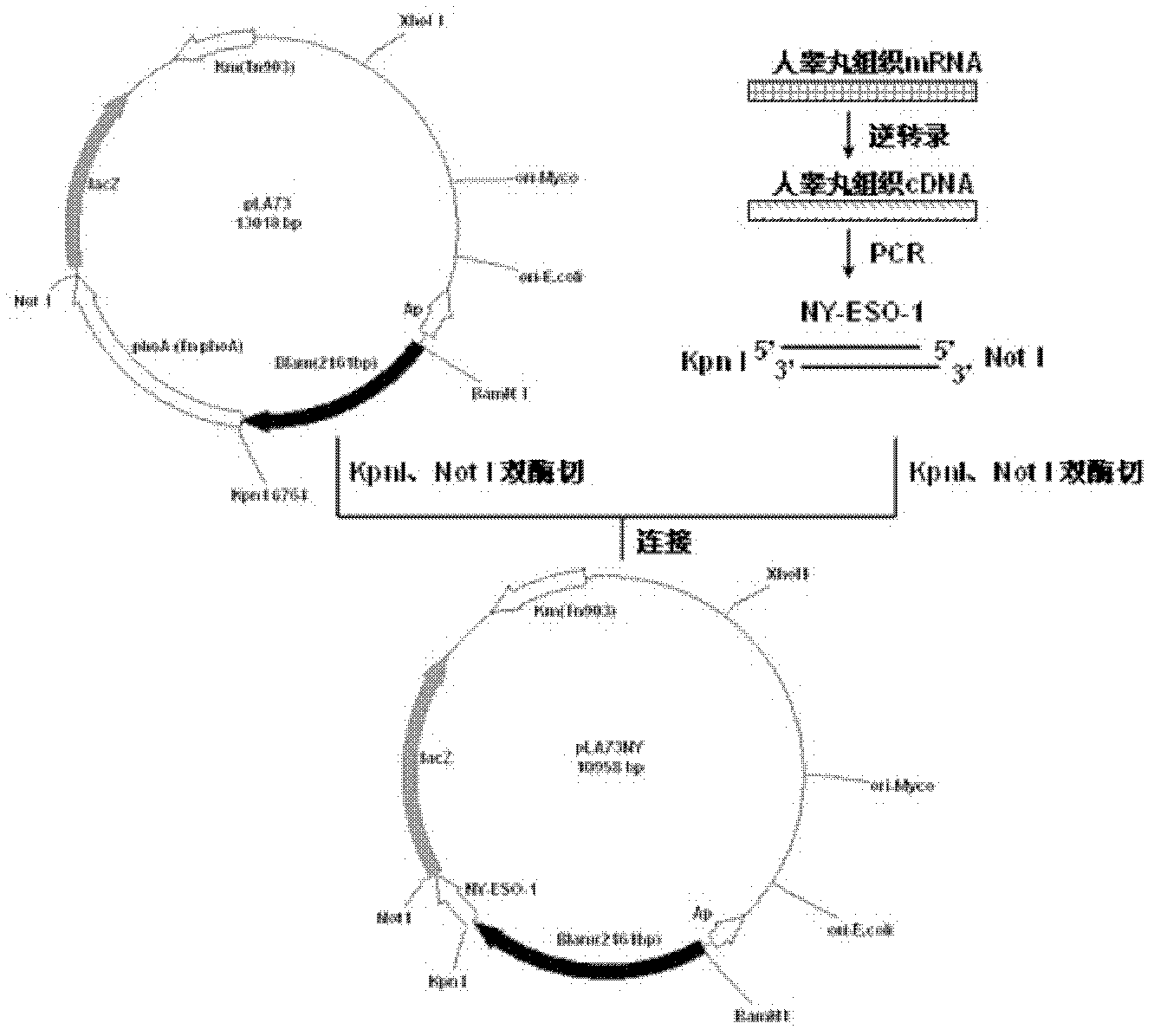
[0136] Example 1. Plasmid (pLA71NY) for cell wall-localized expression of NY-ESO-1 in Mycobacterium smegmatis or pLA73NY recombinant plasmid) construction
[0137] The human testis tissue mRNA was reverse-transcribed into cDNA according to conventional methods, and the NY-ESO-1 gene was amplified by PCR using the human testis tissue cDNA (GenBank number: AJ003149.1, purchased from Shanghai Shenergy Biology Co., Ltd.) as a template. The sequences of the forward primer and the reverse primer are shown in SEQ ID NO: 9 and SEQ ID NO: 10, respectively. The PCR reaction system is as follows (in μl):
[0138] template
10×Taq buffer
10mMdNTP
wxya 2 o
Taq enzyme
1
1
1
5
1
40
1
[0139] The PCR reaction conditions are: 96°C for 5 minutes → (94°C for 30 seconds → 60°C for 30 seconds → 72°C for 50 seconds) for 30 cycles → 72°C for 5 minutes
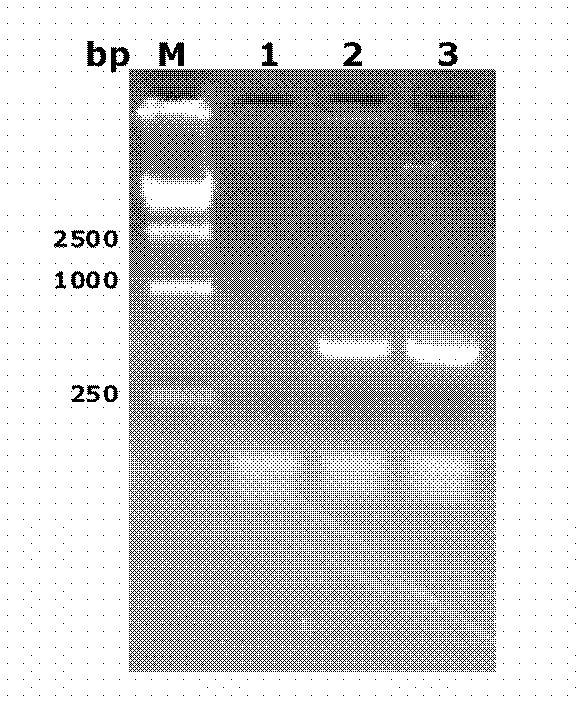
[0140] The target fragment...
Embodiment 2
[0142] Example 2. Construction and verification of recombinant Mycobacterium smegmatis
[0143] 1. Mycobacterium smegmatis mc 2 155 Competent Cell Preparation
[0144] a. inoculate the Mycobacterium smegmatis strain into 3ml of M7H 9-Tween80 medium, and cultivate overnight at 37°C and 250rpm;
[0145] b. Inoculate 150 μl of bacterial liquid into 150 ml of M7H 9-Tween80 medium, and culture overnight at 37°C and 250 rpm;
[0146] c. Measure OD 600 , when it reaches 1.2-1.6, centrifuge at 2500rpm at 4°C for 5min;
[0147] d. Discard the supernatant, resuspend in 150ml ice water, and centrifuge at 2500rpm at 4°C for 5min;
[0148] e. Discard the supernatant, resuspend in 75ml of ice water, and centrifuge at 2500rpm at 4°C for 5min;
[0149] f. Discard the supernatant, resuspend in 10ml 10% glycerol, and centrifuge at 2500rpm at 4°C for 5min;
[0150] g. Discard the supernatant and resuspend in 1.5ml of 10% glycerol to obtain competent cells.
[0151] 2. Electroconversion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com