Method for preparing cyclic carbonate from carbon dioxide and epoxy compound through cycloaddition
A technology for epoxy compounds and cyclic carbonates, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problems of complex preparation process and high catalyst cost, To achieve the effect of good selectivity, low price and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
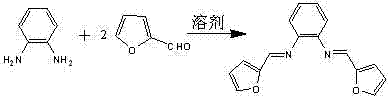
[0021] (1) References Mukhopadhyay M, Reddy M M, Maikap G C, et al. Journal of Organic Chemistry , 1996, 60: 2670~2676, preparation of catalyst ligand bisfural formaldehyde ortho-phenylenediamine by reaction of o-phenylenediamine and furan formaldehyde: take 1.92g (20mmol) 2-furan formaldehyde dissolved in 10mL absolute ethanol, stir at room temperature Next, a solution of 1.08g (10mmol) of o-phenylenediamine dissolved in 10mL of absolute ethanol was slowly added to the 2-furan carboxaldehyde ethanol solution. Continue to stir the reaction at room temperature for 5 hours, let it stand overnight, extract with petroleum ether, and obtain pale yellow needle-like crystals, which are the catalyst ligand bisfurylformaldehyde-o-phenylenediamine. 1 H NMR (400 MHz, CDCl 3 ): δ 7.80,6.24(m, 6H, furan H ), 7.48(s, 2H, -CH=N), 7.46, 7.22(m, 4H, Ph H). Infrared ν (cm -1 ): 1642 (C=N), 1564, 1508, 1392 (aromatic ring), 1287 (C-O-C). Melting point: 94-97°C. Elemental Analysis C 16 h 1...
Embodiment 1
[0027] will be 0.1×10 -3 Put the molar bisfural formaldehyde acetal o-phenylenediamine zinc complex catalyst into a pre-dried 100mL stainless steel autoclave, vacuumize, replace the air in the autoclave with high-purity nitrogen, and add purified propylene oxide into the autoclave with a syringe 0.02 moles, heated up to 85°C, and introduced CO 2 to 1.5MPa, stirred for 10 hours, then lowered the temperature to stop the reaction. After the reaction system is cooled, the remaining gas is released, distilled, and the distillate is collected around 34°C to obtain propylene oxide, and then continue to heat up and distill until there is no distillate, and the obtained colorless liquid is propylene carbonate .
[0028] The yield of propylene carbonate analyzed by gas chromatography was 83%.
[0029] The distillation residue is transferred to an autoclave to be used as a catalyst for the next catalytic reaction.
[0030] The catalyst was directly reused 4 times, and the yields of p...
Embodiment 2
[0033] Will 1×10 -3 Put the molar bisfural formaldehyde acetal o-phenylenediamine zinc complex catalyst into a pre-dried 2L stainless steel autoclave, vacuumize, replace the air in the autoclave with high-purity nitrogen, and add purified propylene oxide into the autoclave with a syringe 10 moles, heated up to 85°C, and introduced CO 2 to 5MPa, stirred for 36 hours, then cooled down to stop the reaction. After the reaction system is cooled, the remaining gas is released, distilled, and the distillate is collected around 34°C to obtain propylene oxide, and then continue to heat up and distill until there is no distillate, and the obtained colorless liquid is propylene carbonate .
[0034] The yield of propylene carbonate analyzed by gas chromatography was 71%.
[0035] The distillation residue is transferred to an autoclave to be used as a catalyst for the next catalytic reaction.
[0036] The catalyst was directly reused 4 times, and the yields of propylene carbonate were ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com