Amino acid modified spinacin derivative and preparation method and application thereof
An amino acid, glycine acyl technology, applied in drug combinations, dipeptide components, blood diseases, etc., can solve problems such as bioavailability limitation and poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
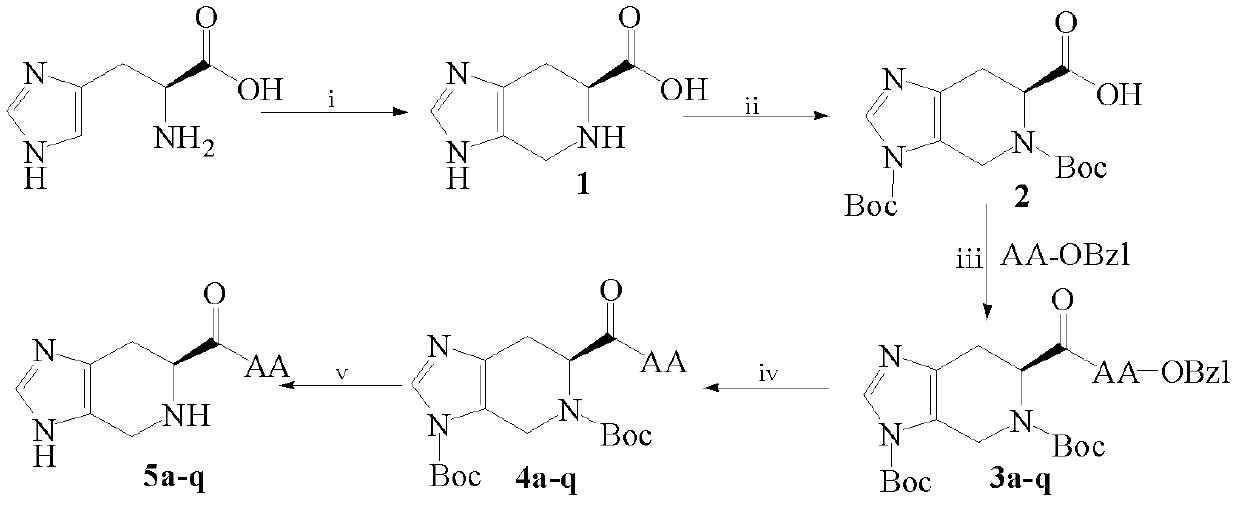
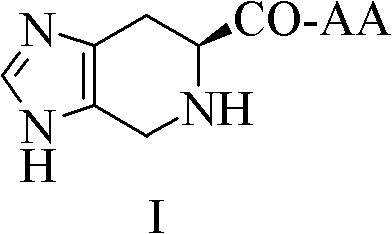
[0020] Embodiment 1 (6S)-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid synthesis
[0021] Put 10g of L-His in a 250ml eggplant-shaped bottle, dissolve it with a mixed solution of 80ml of distilled water and 20ml of formaldehyde, add 1ml of concentrated H 2 SO 4 , react in an oil bath at 60-70°C for 12h, cool to room temperature, add concentrated ammonia water dropwise under an ice bath to adjust the pH to 7-8, a large amount of colorless precipitate precipitated, and filtered to obtain 10.5g (97%) of the title compound as a colorless solid. ESI-MS(m / z): 167[M+H] +
Embodiment 2
[0022] Example 2 (6S)-3,5-two (tert-butoxycarbonyl)-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid synthesis
[0023] Dissolve 1.67g (10mmol) (6S)-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid in 2N aqueous sodium hydroxide solution under ice-bath conditions , take 5.23g (24mmol) (Boc) 2 O, dissolved with dioxane, added to the reaction solution. Stir at room temperature, TLC monitors the disappearance of the reaction raw material point, after the completion of the reaction, use saturated KHSO 4 The pH of the aqueous solution was adjusted to neutral, and concentrated under reduced pressure to remove dioxane. The aqueous layer was saturated with KHSO 4 The aqueous solution was acidified to pH=2, extracted with ethyl acetate and concentrated under reduced pressure to remove dioxane, the aqueous layer was extracted and washed with ethyl acetate (30ml×3), and the combined ethyl acetate layers were washed repeatedly with saturated NaCl until neutr...
Embodiment 3
[0024] Example 3 (6S)-3,5-bis(tert-butoxycarbonyl)-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-formyl-amino acid benzyl General method for the preparation of esters (3a-q)
[0025] Weigh 1.101g (3mmol) (6S)-3,5-bis(tert-butoxycarbonyl)-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxyl Dissolve the acid in a 100ml eggplant-shaped bottle with a small amount of anhydrous tetrahydrofuran (THF), and then add 486mg (3.6mmol) of 1-hydroxybenzotriazole (HOBt) and 768mg (3.6mmol) of di Cyclohexylcarbodiimide (DCC), after activating for 30min, weigh AA-OBzl (3.6mmol) in a 25ml small Erlenmeyer flask, suspend it with anhydrous tetrahydrofuran (THF), and use N-methylmorpholine (NMM) Adjust the pH to neutral, then drop the suspension into the reaction solution, finally adjust the pH value of the reaction solution to about 8 with NMM, react overnight at room temperature, monitor by TLC until the raw material spots disappear, filter to remove dicyclohexyl urea ( DCU), the filtrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com