Naphthyl anthracene-substituted dibenzothiophene sulphone organic semiconductor material and preparation method and application thereof
A benzothiophene sulfone and organic semiconductor technology, which is applied in the field of naphthyl anthracene substituted dibenzothiophene sulfone organic semiconductor materials and their preparation fields, can solve the problems of low luminous efficiency, weak luminous intensity, poor carrier transport performance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
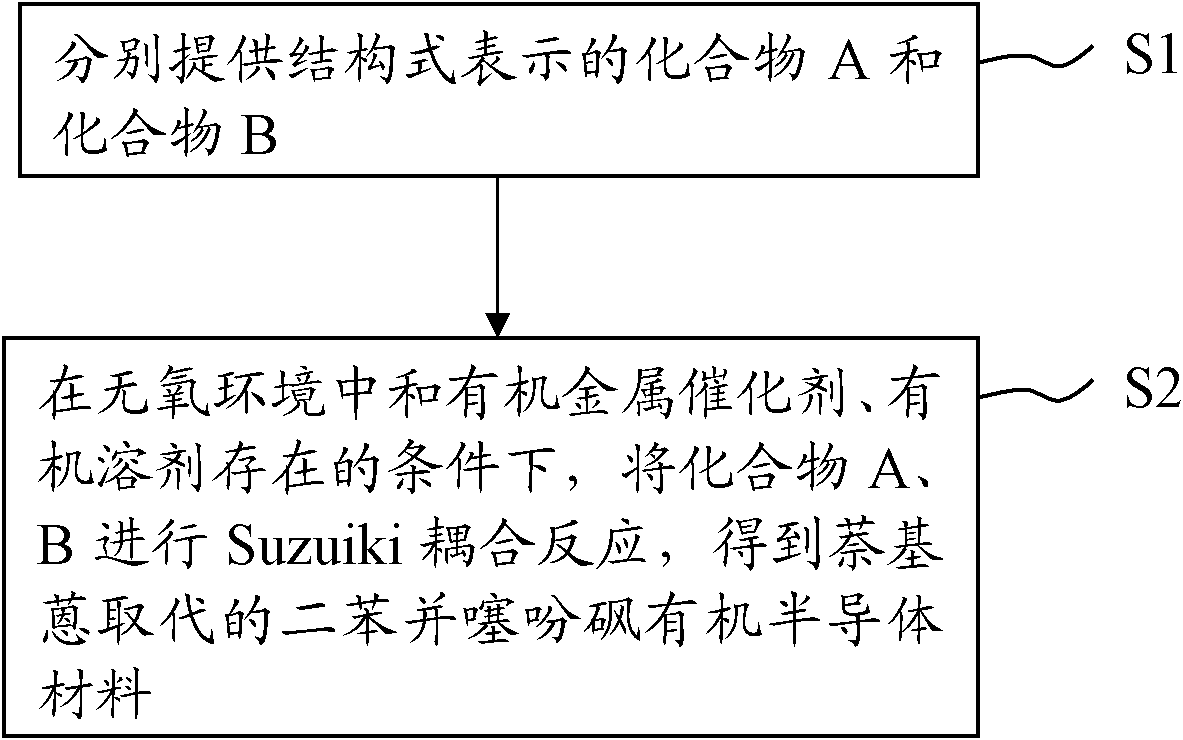
[0031] The embodiment of the present invention also provides a preparation method of the above-mentioned naphthylanthracene-substituted dibenzothiophene sulfone organic semiconductor material. For the process flow of the method, please refer to figure 1 . The preparation method of the naphthylanthracene-substituted dibenzothiophene sulfone organic semiconductor material includes the following steps:
[0032] S1: Provide compound A and compound B represented by the following structural formulas respectively, where R is C 1 ~C 6 The alkyl group,
[0033]
[0034] S2: Suzuiki coupling reaction of compound A and compound B in the presence of an organometallic catalyst and an organic solvent in an oxygen-free environment to obtain the naphthylanthracene-substituted dibenzothiophene represented by the following general structural formula (I) Sulfone organic semiconductor material; the Suzuiki coupling reaction formula of step S2 can be expressed as follows:
[0035]
[0036] Specifically,...
Embodiment 1
[0047] In this embodiment, the naphthylanthracene-substituted dibenzothiophene sulfone organic semiconductor material is the naphthylanthracene-substituted dibenzothiophene sulfone organic compound 2,7-bis(1-methyl-10-(naphth-2-yl)anthracene -9-yl) dibenzothiophene sulfone (DNMAFSO) and its preparation method, its structural formula is as follows I 1 Shown:
[0048]
[0049] The preparation steps of the above polymer are as follows:
[0050] S11: Provide compounds A and B represented by the following structural formulas respectively,
[0051]
[0052] Among them, the specific preparation steps of compound A, 2,7-dibromodibenzothiophene sulfone are: dissolving 4mmol of dibenzothiophene sulfone in 30ml of concentrated H 2 SO 4 Add 8.2mmol NBS at room temperature, stir the reaction, wait for 24h, then pour the reaction solution into water, filter with suction, wash with water and methanol, collect the solid, and then recrystallize the solid from chlorobenzene to obtain a colorless Need...
Embodiment 2
[0060] In this embodiment, the naphthylanthracene-substituted dibenzothiophene sulfone organic semiconductor material is the naphthylanthracene-substituted dibenzothiophene sulfone organic compound 2,7-bis(1-ethyl-10-(naphth-2-yl)anthracene -9-yl) dibenzothiophene sulfone (DNEtAFSO) and its preparation method, its structural formula is as follows I 2 Shown:
[0061]
[0062] The preparation steps of the above polymer are as follows:
[0063] S21: Provide compounds A and B represented by the following structural formulas respectively,
[0064]
[0065] Wherein, the method for obtaining compound A, namely 2,7-dibromodibenzothiophene sulfone, is the same as step S11 in Example 1.
[0066] S22: Preparation of 2,7-bis(1-ethyl-10-(naphthalen-2-yl)anthracene-9-yl)dibenzothiophene sulfone (DNEtAFSO), the chemical reaction formula is as follows:
[0067]
[0068] The specific preparation process is as follows: the above compound A (2,7-dibromodibenzothiophene sulfone) 3mmol, compound B (1-ethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com