Organic semiconductor material and preparation method thereof
An organic semiconductor, selected technology, applied in semiconductor/solid-state device manufacturing, semiconductor device, organic chemistry, etc., can solve the problems of blue light emitting material luminous efficiency, poor stable carrier transport performance, etc., to improve solubility and performance. Film-forming properties, improving stability, and achieving the effect of charge balance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
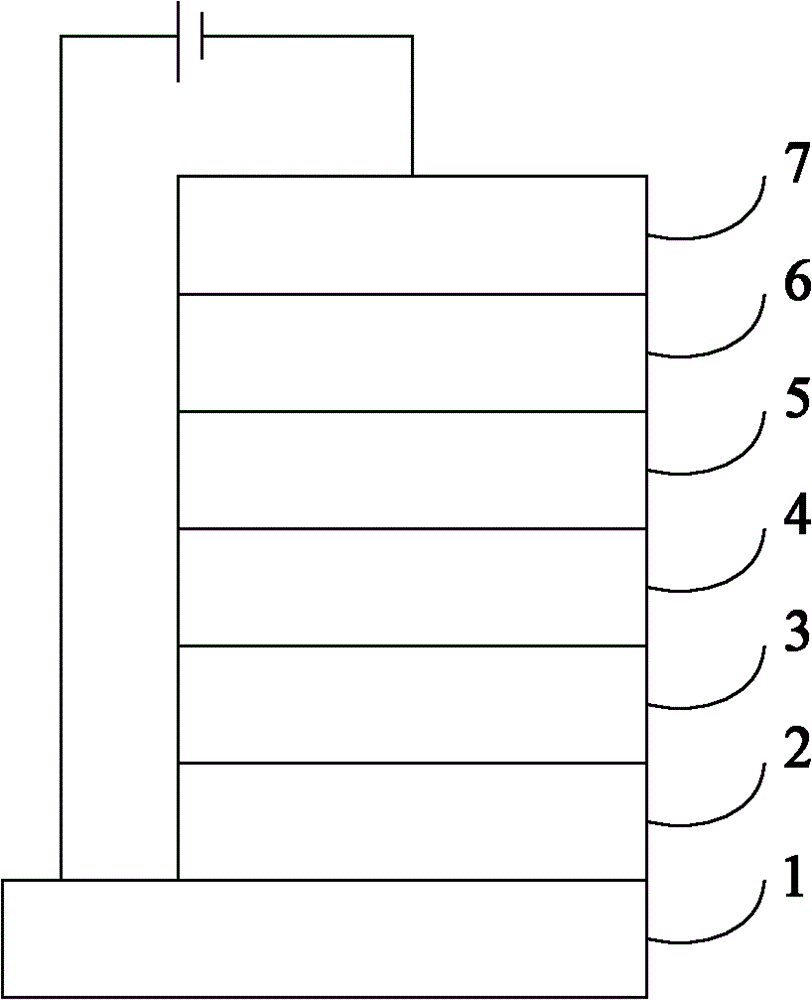
Image
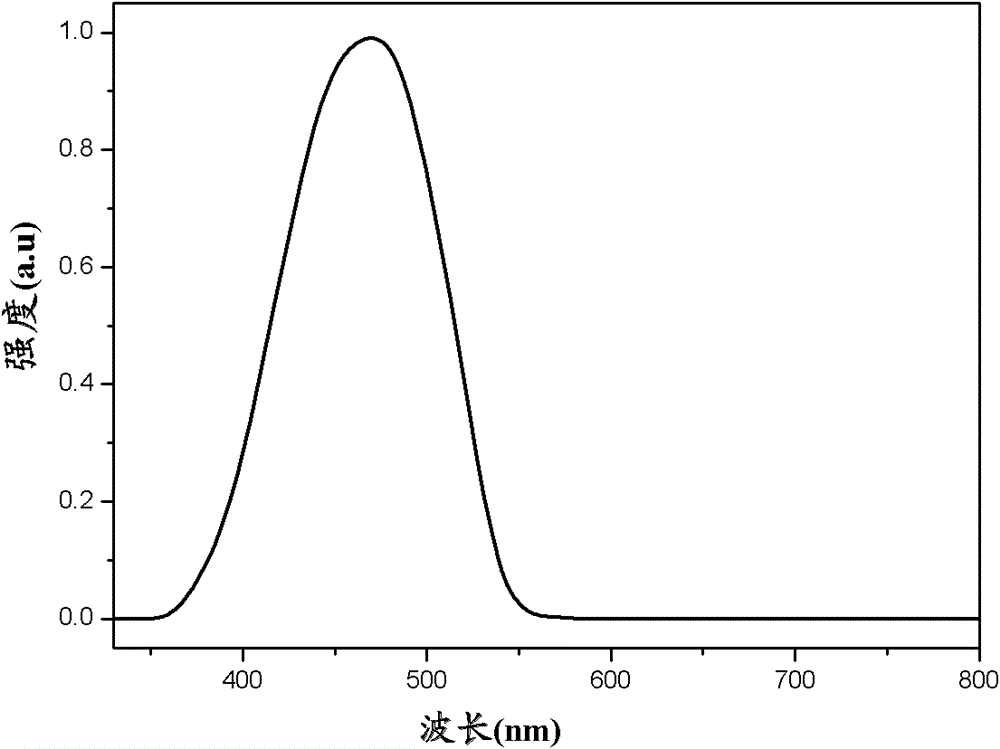
Examples
preparation example Construction
[0023] The embodiment of the present invention further provides a method for preparing the above-mentioned organic semiconductor material, including the following steps:
[0024] Step S01, providing respectively 5-(10-D anthracene-9-yl)benzothiadiazol-2-ylboronic acid of structural formula I or 5-(10-D anthracene-9-yl)benzothiadiazole of structural formula II The tripolyindene monomer of oxazol-2-ylboronic acid ester, structural formula III: structural formula I is: Structural formula II is: Structural formula III is:
[0025] Step S02,
[0026] In an inert atmosphere, combine the tripolyindene monomer of the structural formula III with the 5-(10-D anthracene-9-yl)benzothiadiazol-2-ylboronic acid of the structural formula I or the 5-(10-D Anthracene-9-yl) benzothiadiazol-2-yl borate is dissolved in an organic solvent at a molar ratio of 1:3-6, and a Suzuiki reaction is carried out under catalyst conditions to obtain an organic semiconductor material of the following str...
Embodiment 2
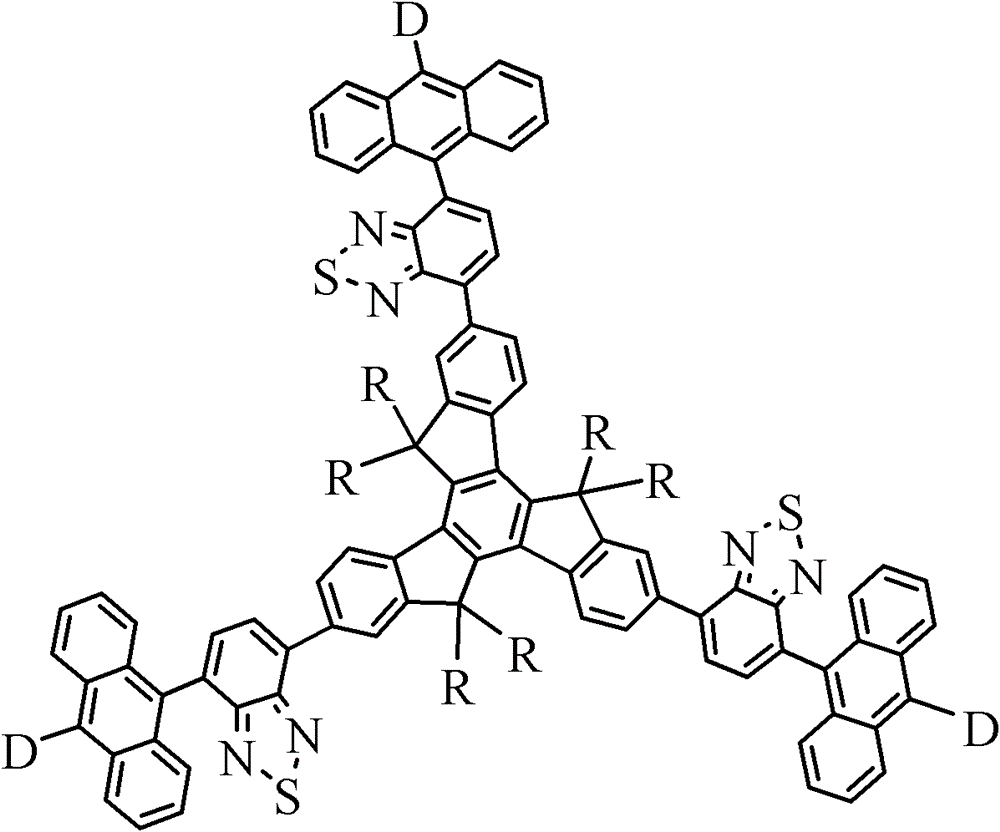
[0067] Example of the present invention 2,7,12-tris(5-(10-n-hexyl anthracene-9-yl)benzothiadiazol-2-yl)-5,5', 10,10', 15,15' -hexaethyltriindene (HABET) has the following structural formula:
[0068]
[0069] Example of the present invention 2,7,12-tris(5-(10-n-hexyl anthracene-9-yl)benzothiadiazol-2-yl)-5,5', 10,10', 15,15' -hexaethyl tripolyindene (HABET) preparation method, comprises the steps:
[0070] Step 1, prepare tripolyindene:
[0071] Add 10 mmol of 1-indanone into a mixed solution containing 8 mL of acetic acid and 4 mL of concentrated hydrochloric acid, heat to 100° C., and stir and reflux for 20 h. After the reaction, the reaction solution was poured into a beaker filled with ice water, and a large amount of solid precipitated immediately. After the precipitate was washed with water, acetone and dichloromethane in sequence, a white solid powder, that is, trisindene, was obtained, with a yield of 91%. EI-MS: m / z 343 (M + ). The reaction formula is express...
Embodiment 2
[0094] Embodiments of the present invention 2,7,12-tris(5-(10-n-hexyloxyanthracene-9-yl)benzothiadiazol-2-yl)-5,5',10,10',15,15 '-hexaethyltripolyindene (HOABET) has the following structural formula:
[0095]
[0096] Embodiments of the present invention 2,7,12-tris(5-(10-n-hexyloxyanthracene-9-yl)benzothiadiazol-2-yl)-5,5',10,10',15,15 '-hexaethyl tripolyindene (HOABET) preparation method, comprises the steps:
[0097] Step 1 refers to step 1 in Embodiment 1;
[0098] Step 2, the preparation of 5-(10-n-hexyloxyanthracene-9-yl)benzothiadiazol-2-ylboronic acid:
[0099] Preparation of 10-n-hexyloxy-9-bromoanthracene:
[0100] Dissolve 15mmol of 10-n-hexyloxyanthracene in 25mL of dichloromethane, and at the same time dissolve 16mmol of NBS in 15mL of dichloromethane, and slowly add the NBS-containing dichloromethane solution at 0°C to the solution containing 10-n-hexyloxy Add 1ml of glacial acetic acid to the dichloromethane solution of base anthracene, and react in the d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com