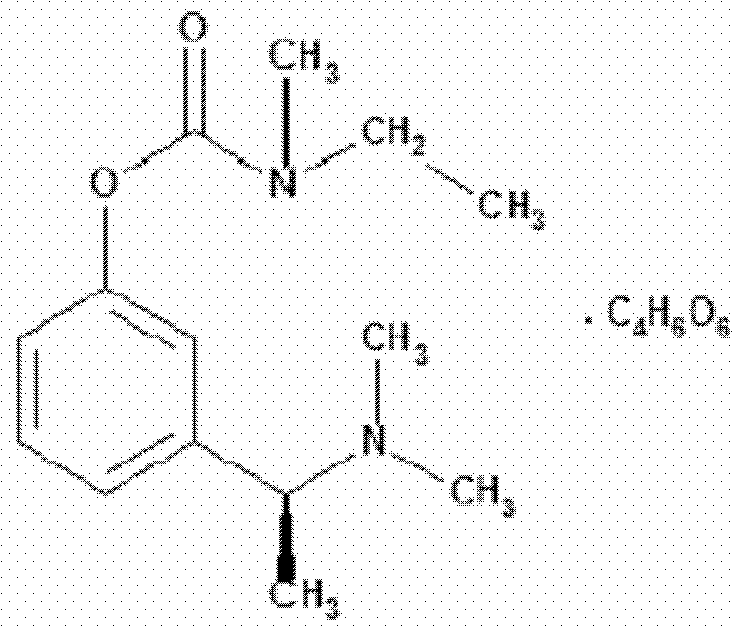
Rivastigmine capsules and preparation method thereof
A technology of rivastigmine bitartrate and capsules, which is applied in the field of rivastigmine bitartrate preparation and its preparation, rivastigmine bitartrate capsule and its preparation field, which can solve the problems of easy deliquescence and difficult storage, etc., and achieve moisture absorption Low, conducive to storage, uniform particle size distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
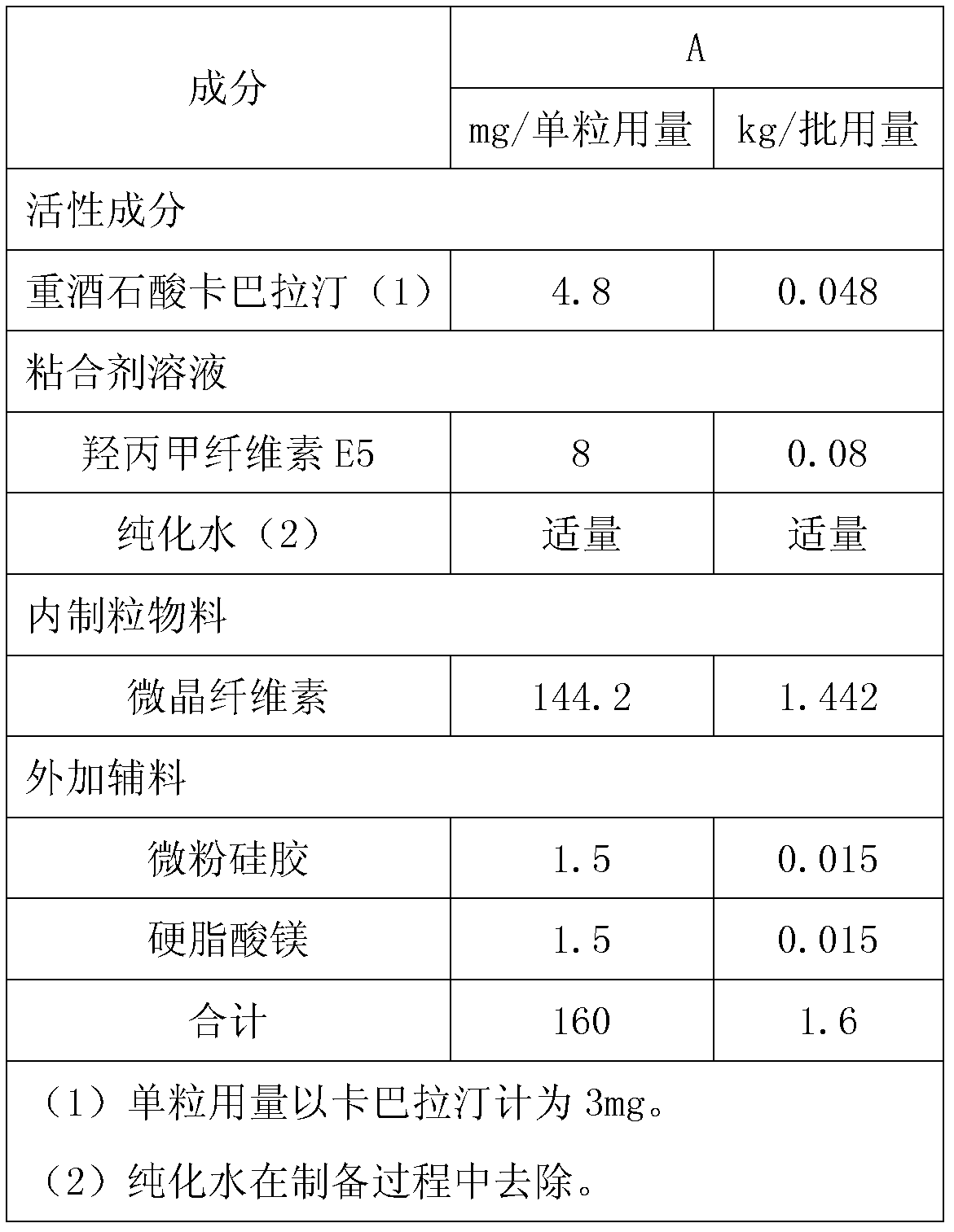
[0032] prescription:
[0033]
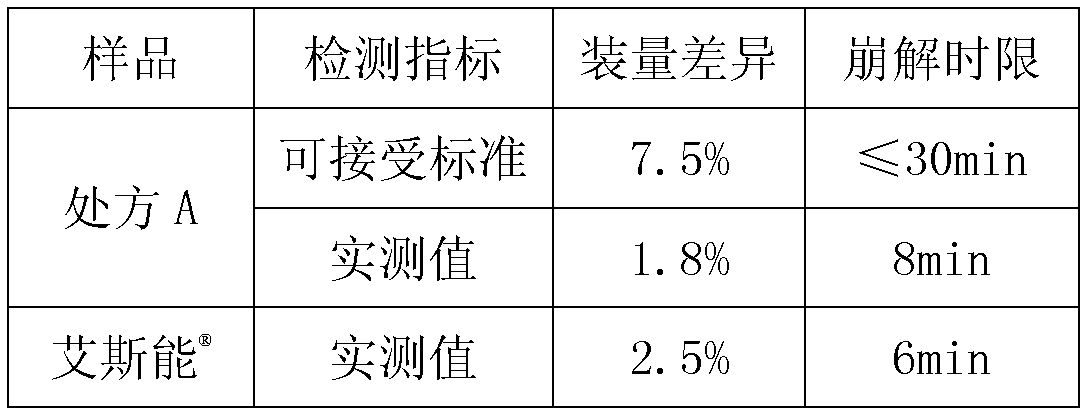
[0034] Preparation: Weigh 0.08kg of hypromellose and add it to a 5L stainless steel bucket, add about 2kg of purified water, stir and dissolve to obtain a 4% hypromellose aqueous solution; weigh 0.048kg of rivastigmine bitartrate and add it to about 0.5kg In the hypromellose aqueous solution, stir to obtain a clear solution; pour 1.442kg of microcrystalline cellulose into the fluidized bed granulator; then spray the hypromellose aqueous solution containing rivastigmine bitartrate on micro Prepare granules on crystalline cellulose, set the inlet air temperature to 50°C; adjust the inlet air temperature to 60°C, and continue to coat the granules with the remaining hypromellose aqueous solution until the weight gain reaches 3.5%. Dry the granules to control the weight loss on drying to less than 2.0% (background temperature 105°C); add additional silicon dioxide and magnesium stearate, and mix well; use hypromellose capsule shells to fill the ca...
Embodiment 2
[0048] prescription:
[0049]
[0050]
[0051]Note: prescription D does not belong to the present invention, here as a comparative example.
[0052] Preparation: Weigh 36g of hypromellose and add it to a 2000ml beaker, add 1800g of purified water to prepare a 2% hypromellose aqueous solution; weigh the batch amount (9.6g) of rivastigmine bitartrate and add it to 360g of hydroxypropyl methylcellulose In the aqueous solution of propyl methylcellulose, stir to obtain a clear solution; the starch1500 and (454.4g) microcrystalline cellulose of the batch amount (90g) are poured into the fluidized bed granulator and mixed; Spray the propyl methylcellulose aqueous solution on the mixture to obtain granules, set the inlet air temperature to 50°C; adjust the inlet air temperature to 60°C, and continue to coat the granules with the remaining hypromellose aqueous solution until the weight gain is 4.5 %. The granules are dried, and the weight loss on drying is controlled to 1.5% (...
Embodiment 3
[0058] prescription:
[0059]
[0060] Preparation: Weigh hypromellose according to the prescription in the above table and add them into stainless steel barrels, add purified water to prepare a 2% hypromellose aqueous solution; In the hypromellose aqueous solution of / 9 weight, stir to obtain clear solution; Pour the mannitol and the microcrystalline cellulose of batch amount into the fluidized bed granulator and mix; Then the hypromellose containing rivastigmine bitartrate The cellulose aqueous solution was sprayed on the mixture to obtain granules, and the air inlet temperature was set to 50°C; the air inlet temperature was adjusted to 60°C, and the remaining part of the hypromellose aqueous solution was used to coat the granules until the granule weight increased by about 7%. Dry the granules, and control the loss on drying to 2.5% (background temperature 105°C); add additional silicon dioxide and magnesium stearate, and mix well; use hypromellose capsule shells to fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com