Preparation method for magnesium-aluminium hydrotalcite
A technology of magnesium-aluminum hydrotalcite and aluminum chloride, applied in the direction of alumina/aluminum hydroxide, etc., can solve the problems of large metal salt, high cost, low atomic utilization rate, etc., and achieve high atomic utilization rate and reduce industrialization cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of hydrotalcite with a magnesium-aluminum ratio of 2:1
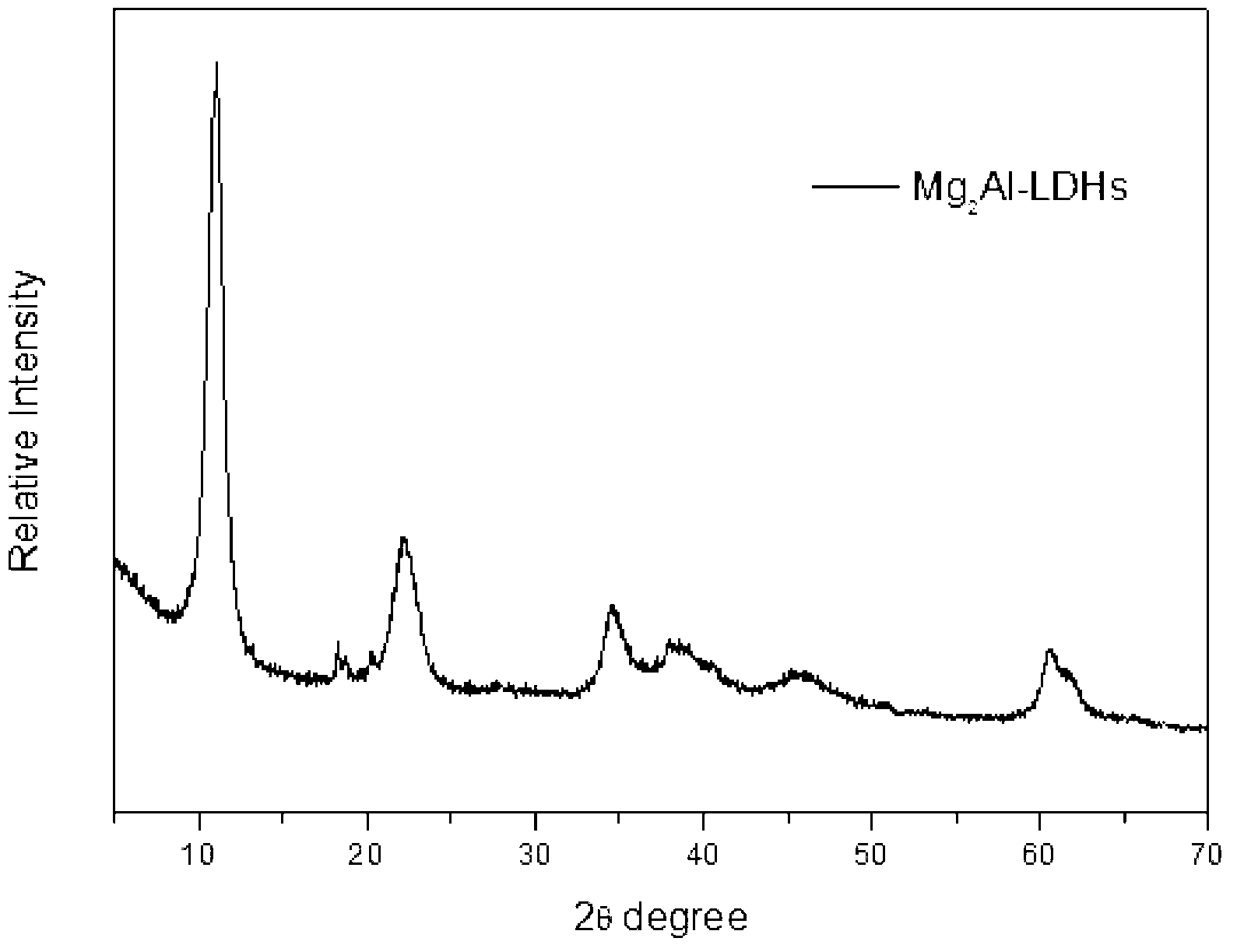
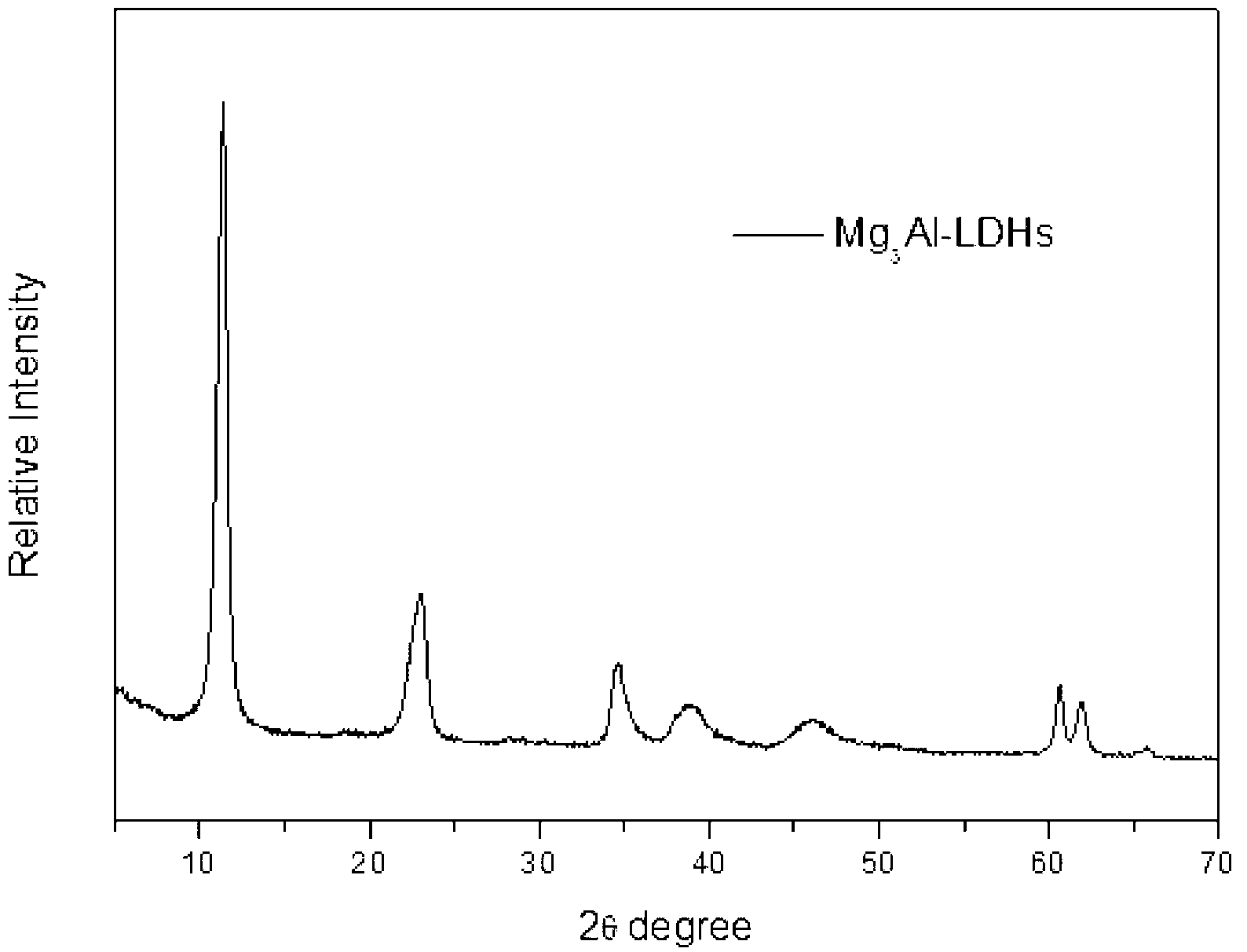
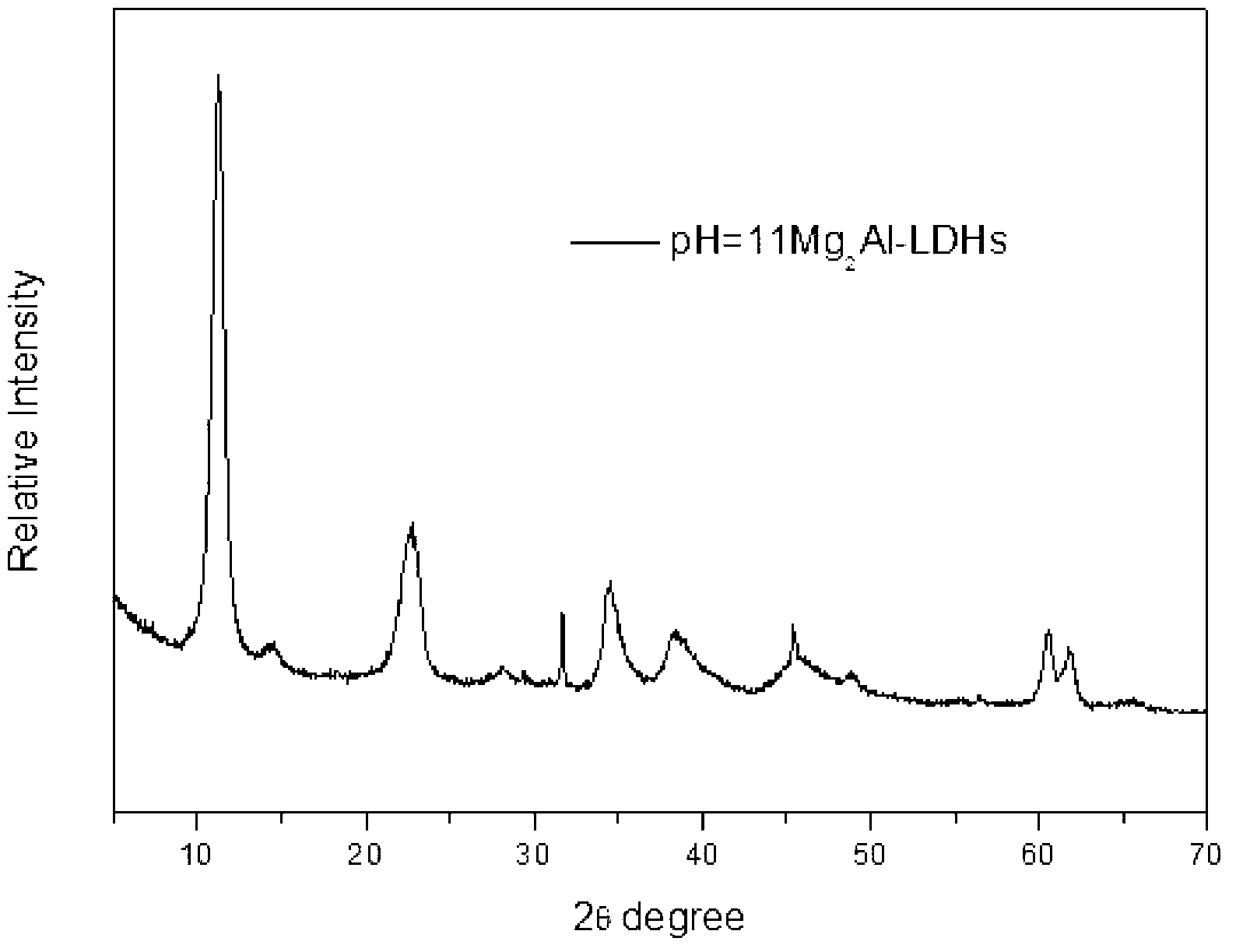
[0035] Take 8.0gMgO and 24.0gAlCl 3 ·H 2 O, AlCl 3 ·H 2 Dissolve O in 200ml of distilled water, add MgO with stirring at 80°C, and adjust the pH after reacting for 2.5 hours. Take 2mol / L NaOH solution to adjust pH=9.18. After continuing to react for two and a half hours, it was crystallized at 60°C for 18 hours. After suction filtration and washing for 4 times, drying at 60° C. for 12 hours and grinding to obtain magnesium aluminum hydrotalcite. See XRD diagram figure 1 .
Embodiment 2
[0036] Example 2: Preparation of hydrotalcite with a magnesium-aluminum ratio of 2:1
[0037]Take 8.0gMgO and 24.0gAlCl 3 ·H 2 O, AlCl 3 ·H 2 Dissolve O in 400ml of distilled water, add MgO with stirring at 60°C, and adjust the pH after reacting for 2.5 hours. Take 2mol / L NaOH solution NaOH to adjust pH=9.18. After continuing the reaction for two and a half hours, it was crystallized at 80°C for 12 hours. After suction filtration and washing for 4 times, drying at 80° C. for 12 hours and grinding to obtain magnesium aluminum hydrotalcite.
Embodiment 3
[0038] Example 3: Preparation of hydrotalcite with a magnesium-aluminum ratio of 2:1
[0039] Take 4.0gMgO and 12.0gAlCl 3 ·H 2 O, AlCl 3 ·H 2 O was dissolved in 400ml of distilled water, and MgO was added with stirring under heating at 40°C, and the pH was adjusted after reacting for 4 hours. Take 2mol / L NaOH solution NaOH to adjust pH=9.18. After continuing the reaction for three hours, the crystallization was carried out at 60° C. for 24 hours. After suction filtration and washing for 4 times, drying at 60° C. for 20 hours and grinding to obtain magnesium aluminum hydrotalcite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com