Fumagillol-type compounds and methods of making and using same
A compound and composition technology, applied in the field of pharmaceutical use and its preparation, can solve problems such as harmful side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Compound A was tested in the MetAP2 enzyme assay. Table 1 indicates the inhibition results for various concentrations:
[0087] 1hr 200nM
[0088] In cellular assays, inhibition of MetAP2 was <10 nM.
Embodiment 2
[0090] Weight loss studies were performed in obese mice. The mice in this study were not genetically obese, but were induced to become obese by a high-fat diet before and during the study. Twelve-week-old C57BL / 6NTac mice maintained on a 60% fat diet before and during the study were divided into 7 groups of 8 mice each. The average mouse weight at the start of the study was approximately 47 g.
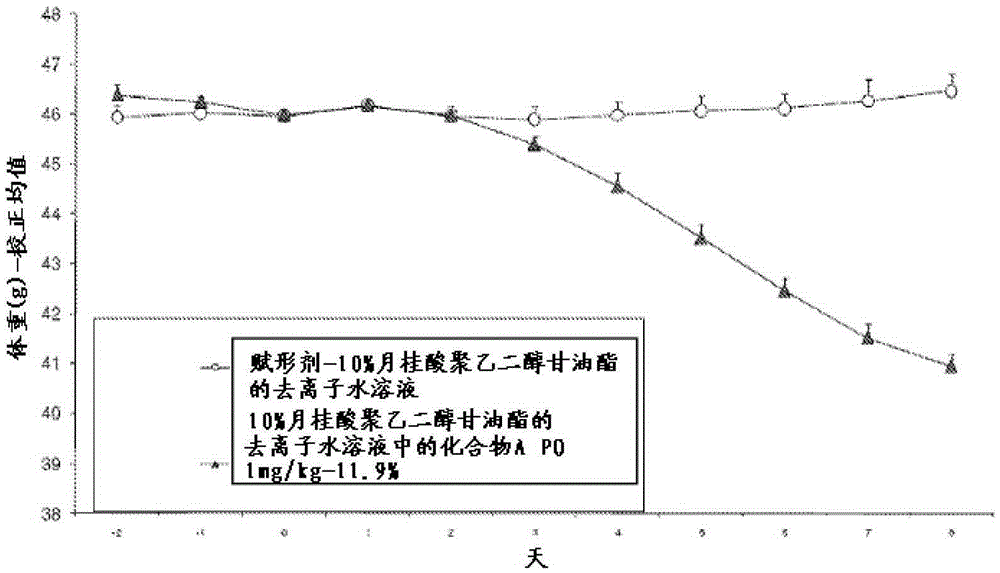
[0091] Mice were administered 1.0 mg / kg of Compound A in 10% macrogolglyceride laurate in deionized water. The mice received the drug once a day for 7 consecutive days. 11.9% weight loss in one week.
Embodiment 3
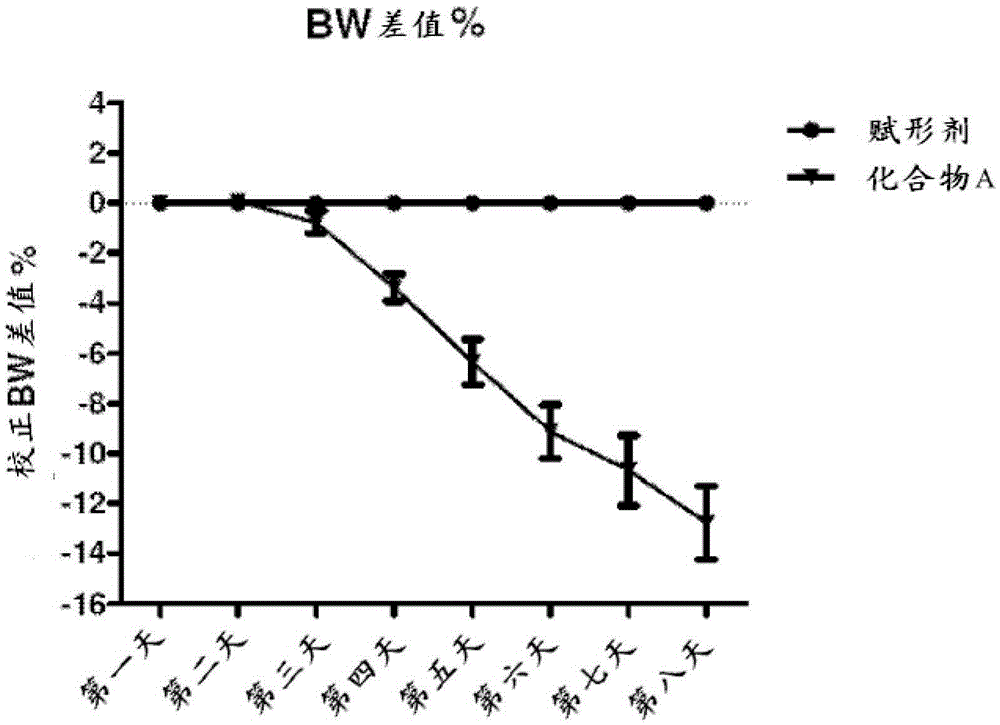
[0093] Three studies were performed using 18-20 week old C57BL / 6 mice. Study A was a 7-day screen consisting of feeding 18-20 week-old C57BL / 6 mice a 45% kcal fat diet for 14 weeks with 10% macrogolglyceride laurate in water or containing The same vehicle treatment with the same vehicle in the amount of Compound A at a daily dose of 1 mg / kg for 7 days. figure 1 The body weight loss effect of 1 mg / kg dose was shown.
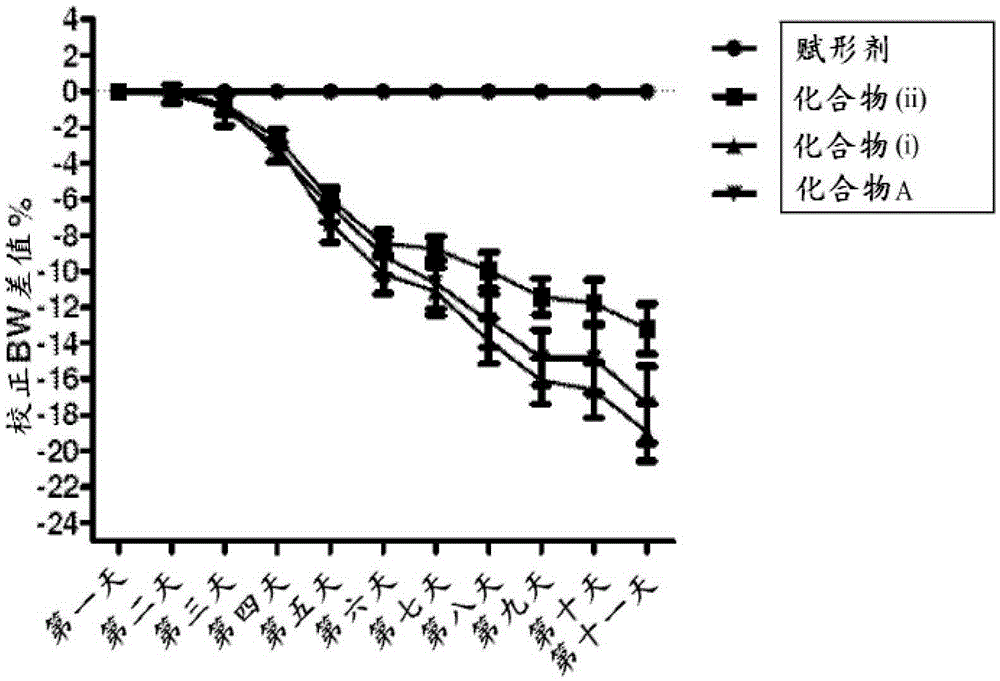
[0094] Study B was a 10-day study that included 20-week-old C57BL / 6 mice receiving a 60% kcal fat diet for 14 weeks with either 10% DMSO or a drug containing 10% DMSO that provided a daily dose of 3 mg / kg Compound A for 10 consecutive days. The amount of drug treated with the same excipients. figure 2 The 7-day PO potency of Compound A at 3 mg / kg is depicted.
[0095] Study C was a 10-day study consisting of 20-week-old C57BL / 6 mice receiving a 60% kcal fat diet for 14 weeks with 10% DMSO or with 1 mg / kg or 3 mg / kg of compound 6-O per day. - (4-Dimethylamino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com