Application of cellulase in soluable and secretion expression of recombinant protein in escherichia coli
A kind of Escherichia coli, recombinant protein technology, applied in the application field of cellulase protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
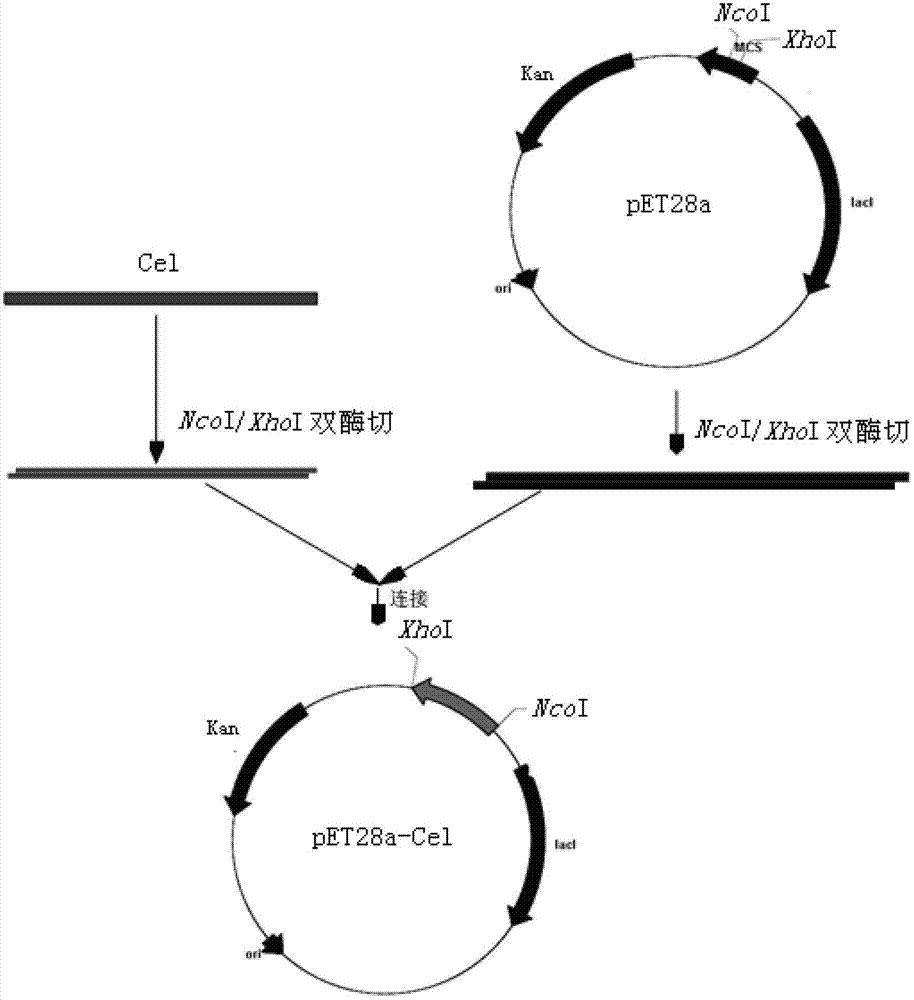
[0059] Embodiment 1: Construction of high-efficiency expression vector of cellulase in Escherichia coli
[0060] 1. Strains and plasmids
[0061] Escherichia coli DH5α (E.coli DH5αLacZΔM15 hsdR recA)
[0062] Escherichia coli BL21(DE3) (E.coli BL21(DE3))
[0063] Escherichia coli BL21(DE3) / pLysS (E. coli BL21(DE3) / pLys)
[0064] Plasmid pET28a
[0065] The above strains and plasmids were purchased from Novagen.
[0066] 2. Enzymes and Reagents for Molecular Cloning
[0067] Restriction enzymes NcoI, BamHI; Pfu DNA polymerase; T4 ligase; protein molecular weight standards were purchased from MBI-Fermentas.
[0068] Agarose Gel DNA Purification Kit, DNA Fragment Purification Kit were purchased from OMEGA Ltd.
[0069] TIANprep Mini Plasmid Kit was purchased from TIANGEN.
[0070] Nucleic acid molecular weight standard 1Kb Marker was purchased from BioLab.
[0071] 3. Method
[0072] Using Bacillus sp.Z-16 genomic DNA as a template, use primers Cel-F / Cel-R to amplify t...
Embodiment 2
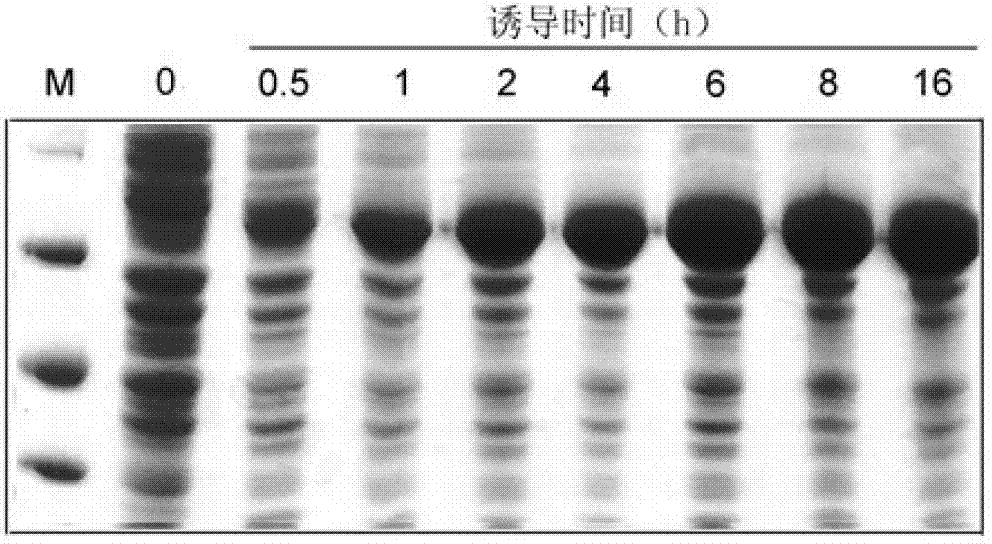
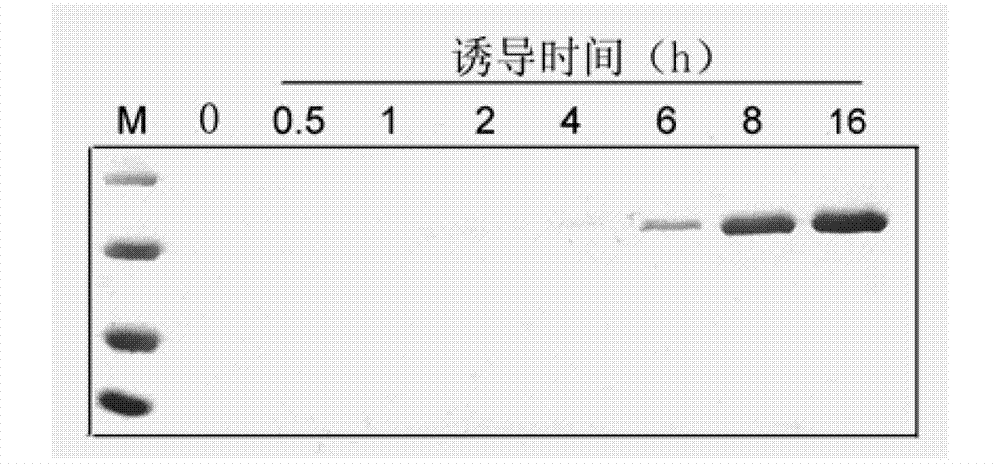
[0090] Example 2: Cellulase Cel is highly expressed and secreted in Escherichia coli
[0091] 1. SDS-PAGE reagent
[0092] Preparation of polyacrylamide gel:
[0093] Liquid A: acrylamide stock solution (30% w / v acrylamide, 0.8% w / v bisacrylamide), take 29.2g acrylamide, 0.8g
[0094] Bisacrylamide, ddH 2 O to 100mL, store at 4°C.
[0095] Solution B: Separating gel buffer, 1.5MTris-HCl (PH8.8) 0.4%SDS. Store at 4°C.
[0096] Solution C: stacking gel buffer, 1MTris-HCl (PH6.8) 0.4%SDS. Store at 4°C.
[0097] 10% ammonium persulfate: take 0.1g ammonium persulfate and add 1mL ddH 2 O, 4°C.
[0098] TEMED: purchased from Sigma, T8133
[0099] Mix the above solutions according to Table 2.1, prepare 12% separating gel and 5% stacking gel, pour into the gel plate, and use it for electrophoresis after polymerization.
[0100] Table 2.1 Polyacrylamide gel formula
[0101]
[0102] Electrophoresis buffer:
[0103] 10×Tris-glycine buffer: 144g glycine, 30g Tris, 10% SDS. ...
Embodiment 3
[0116] Example 3: Efficient and soluble expression of yeast sugar amidase (Png1p) in Escherichia coli by using the protein of the present invention
[0117] Preferably, the amino acid sequence of the catalytic domain of the cellulase is a fusion protein, and the yeast sugar amidase (Png1p) protein is taken as an example to verify the feasibility of the system of the present invention.
[0118] The N-terminal catalytic domain of Cellulase (abbreviated as Cel-CD) is the N-terminal sequence of Cellulase, as shown in SEQ ID NO: 2, consisting of 374 amino acids, and the theoretical length of the gene is 1125bp. Yeast glycoamidase (Png1p) is a protein with 363 amino acids, no signal peptide, and a molecular weight of 42.5kDa. The gene encoding Png1p is located on the left arm of yeast XVI chromosome and consists of 1092 nucleotide sequences. Png1p can be highly expressed in Escherichia coli, but they all exist in the form of inclusion bodies.
[0119] 1. Reagents:
[0120] Buffer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com