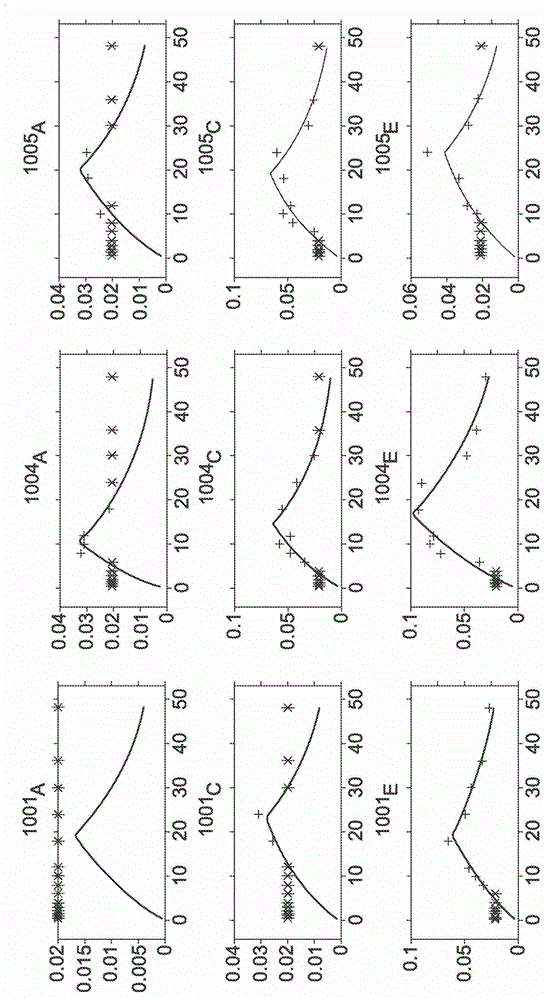
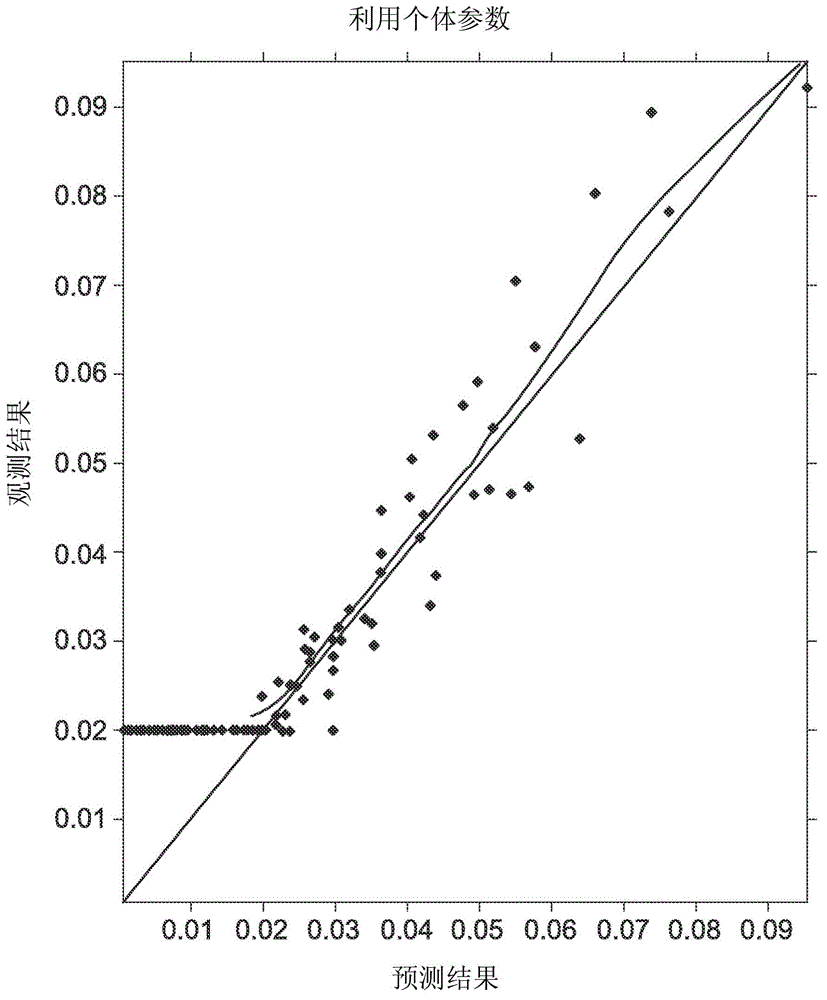
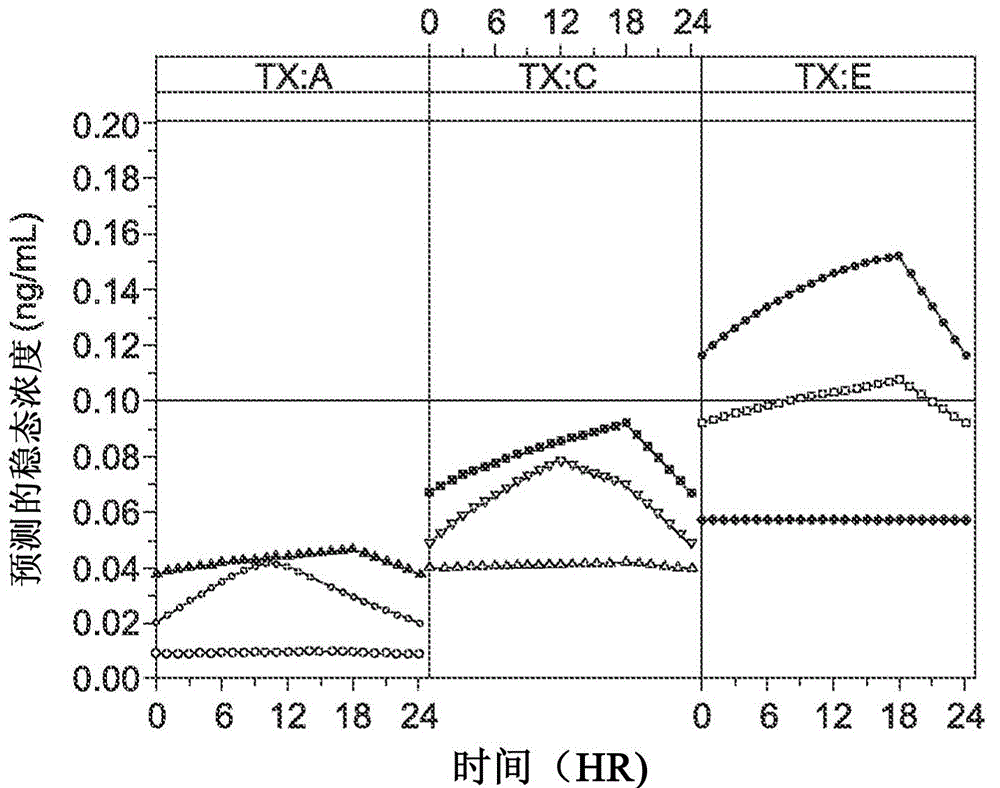
Topical transdermal dexmedetomidine compositions and methods of use thereof
A technology of dexmedetomidine and its composition, which is applied in the field of external transdermal dexmedetomidine composition and its application, and can solve the problem of unsuitable analgesics, no sedative effect, analgesic preparations, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0046] Example 1a - Placebo Transdermal Gel Formulation (Vehicle)
[0047] Exemplary ingredients and methods used to prepare 50 g of Methyl Salicylate / Menthol Hydroalcoholic Clear Gel.
[0048] Table A: 50 g methyl salicylate / menth hydroalcoholic clear gel (RECRO1-45)
[0049]
[0050] Table B: Preparation of 50 g methyl salicylate / menth hydroalcoholic clear gel (RECRO1-45)
[0051]
[0052]
[0053] In this example, the inventors commented as follows: 1) pH = 0.592g, and a large amount of white mass (white mass) was produced after adding water; and 2) Adding water to rehydrate or reconstitute the gel also produced precipitation . The precipitation effect is thought to result from the insolubility of methyl salicylate after more water is added. Furthermore, the occurrence of precipitation is at least partly pH dependent.
Embodiment 1b
[0054] Example 1b - Transdermal Dexmedetomidine Gel Formulation
[0055] Table C: 50 g gel formulation (RECRO1-53) at a concentration of [0.5 mg / g Dex HCl]
[0056] Component No.
[0057] Table D: Preparation of 50 g gel formulation (RECRO1-53) at a concentration of [0.5 mg / g Dex HCl]
[0058] steps
[0059] In the method of this example, several observations were made on step B (before adding component No. 2 dexmedetomidine hydrochloride): 1) the gel preparation became cloudy; The viscosity of the gel obtained in step B is reduced compared to the gel. To resolve the turbidity that occurred in 1, triethanolamine was added and this worked. In this example, triethanolamine was added at a ratio of 0.463 g of triethanolamine (identified as batch number XT0007) to 32.532 g of the gel obtained in step C.
Embodiment 2、3 and 4-De
[0060] Example 2, 3 and 4 - Dex HCl (dexmedetomidine hydrochloride) topical (topical) cream formulation
[0061] The use of a base placebo cream formulation to prepare an active dexmedetomidine hydrochloride transdermal formulation is further described herein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com