Moisture protection method of traditional Chinese medicine extract and solid preparation obtained by traditional Chinese medicine
A technology for solid preparations and extracts, applied in the field of solid preparations of traditional Chinese medicine, can solve the problems of low moisture-proof efficiency and large dosage of auxiliary materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
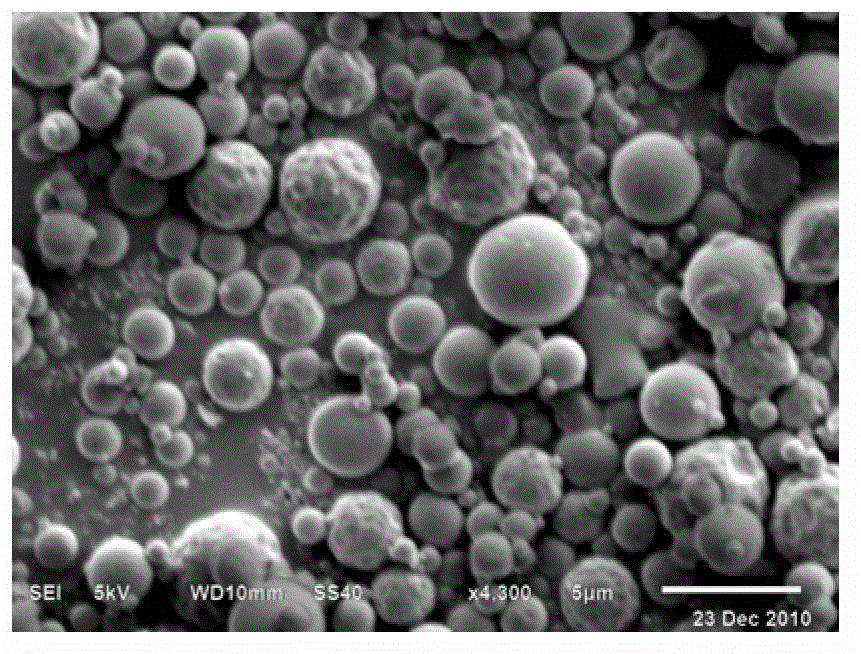
[0049] Example 1 The effect of leucine on yield and fluidity
[0050] Shuanghuanglian extract was prepared according to the preparation method described in "Shuanghuanglian Injection" on page Z11-38 of "Ministry Standards" WS3-B-2104-96, and accurately weighed 2g of this extract in four parts, each placed in 70ml volumetric flask, respectively, ultrasonically dissolved in water to obtain the extract solution of Shuanghuanglian. Weigh 0.06g, 0.1g, 0.2g, 0.4g of leucine, and dissolve them in about 10ml of water, respectively, to prepare leucine aqueous solutions of different concentrations. The aqueous solutions of leucine of various concentrations were added to the four above-mentioned aqueous solutions of Shuanghuanglian extracts, sonicated to make them mixed, and then the volume was fixed to 100ml for use. The aqueous solution containing Shuanghuanglian extract and different concentrations of leucine was spray-dried under the following conditions: an inlet temperature of 100°C,...
Embodiment 2
[0055] Example 2 Effect of Leucine on Hygroscopicity and Particle State after Hygroscopicity
[0056] A Hydrosorb1000 water adsorption tester was used to measure the moisture absorption and desorption percentage change curve of each sample in Example 1. Precisely weigh about 100mg of each sample and place it in the glass tube of the water adsorption tester. At a temperature of 25.0±0.5℃ in the water bath, determine the moisture absorption and desorption percentage change curve of each sample under the change of relative humidity RH10%~90%. figure 2 . Draw the tangent line of the curve from the two ends of each adsorption curve. The abscissa of the intersection of the two tangent lines is the critical relative humidity CRH. The critical relative humidity of each sample particle is 65% to 70%. The addition of auxiliary materials can increase the CRH of the particles, and As the proportion of addition increases. Analyzing the desorption curve of each sample, it can be seen that th...
Embodiment 3
[0061] Example 3 Aerodynamic properties test of spray-dried particles of Shuanghuanglian (the effect of leucine in promoting particle dispersion and inhalation)
[0062] In Comparative Example 1, the difference between the dispersibility and inhalation of the dry powder inhalant prepared by spray-drying blank particles of Shuanghuanglian and adding 10% leucine group particles. Take the Shuanghuanglian spray-dried blank group particles and the 10% leucine group particles in Example 1, respectively Dry powder inhalation lactose is mixed in a ratio of 1:3 according to the principle of equal volume addition, and loaded in No. 3 HPMC capsule to obtain a dry powder inhalant.
[0063] The inhalation evaluation of dry powder inhalants was carried out using the Next Generation Pharmaceutical Impactor (NGI), referring to the 2007 edition of the British Pharmacopoeia Appendix VII F. Apparatus E. Specifically, after assembling the NGI according to the instructions, adjust the pumping speed to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com