Dual-functional probe and preparation method and application in detection of G-quadruplex structure thereof
A dual-function, quadruplex technology, applied in biochemical equipment and methods, chemical instruments and methods, and microbial determination/inspection, etc., can solve the problems of high price and cannot be widely used, etc. Easy to store effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
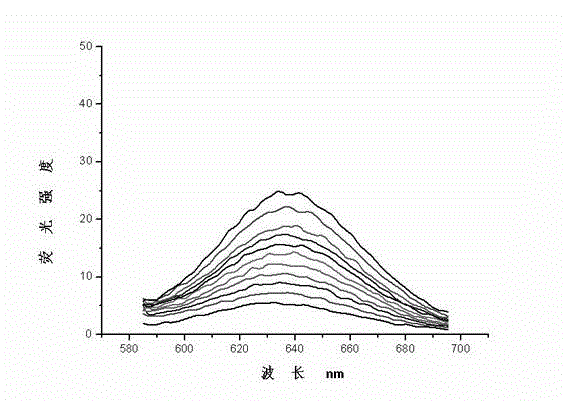
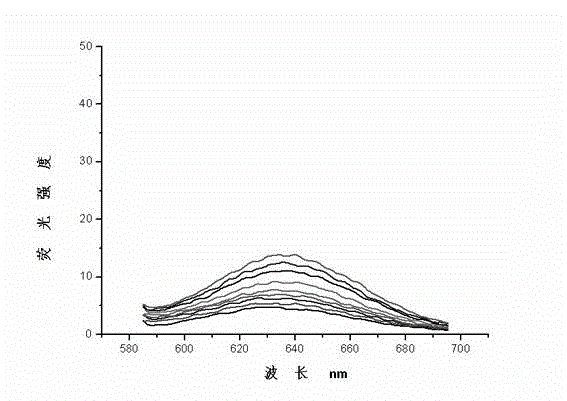
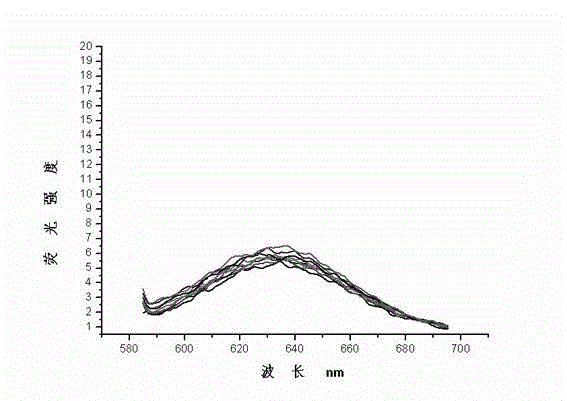
Image
Examples
Embodiment 1
[0055] Embodiment one : Synthesis of Compound 2
[0056] Dissolve 2.01g of 4-diethylamino salicylaldehyde in 30 mL of absolute ethanol, add 3.20 g of diethyl malonate and 1 mL of piperidine, and react at 80°C for 6 h. Then distill off the solvent, add 20 mL of acetic acid and 20 mL of concentrated hydrochloric acid, and continue to reflux for 6 h. After cooling to room temperature, pour the reaction solution into ice water, adjust the pH to 5 with sodium hydroxide solution, and precipitate a large amount of precipitates. Filter and dry to obtain the crude product. Purification by silica gel chromatography using petroleum ether / ethyl acetate (volume ratio 1 / 10) as the eluent gave 0.81 g of pure product 2 with a yield of 37.3%: 1 H NMR (400 MHz, CDCl 3 )δ7.53 (d, J = 9.3 Hz, 1H), 7.24 (d, J = 8.8 Hz, 1H), 6.56 (dd, J = 8.8, 2.1 Hz, 1H), 6.49 (d, J = 1.6 Hz, 1H), 6.03 (d, J = 9.3 Hz, 1H), 3.41 (q, J = 7.1 Hz, 4H), 1.21 (t, J = 7.1 Hz, 6H). ESI-MS m / z: 218.1 [M+ H] + .
Embodiment 2
[0057] Embodiment two : Synthesis of compound 3
[0058] Under nitrogen protection, 1.5 mL POCl 3 Add it dropwise to 1 mL DMF, and stir at room temperature for 20 min. Then 0.77 g of 2 dissolved in 4 mL DMF was added dropwise to the above mixture, reacted at 60 oC for 10 h, cooled to room temperature, poured the reaction solution into ice water, adjusted the pH to neutral with sodium hydroxide solution, and reduced Pressure suction filtration, rinse with water and ethanol several times, and dry in vacuo to obtain 30.50 g of orange-yellow solid, yield 58.3%: 1 H NMR (400 MHz, CDCl 3 )δ10.13 (s, 1H), 8.26 (s, 1H), 7.41 (d, J = 9.0 Hz, 1H), 6.64 (dd, J = 9.0, 2.5 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 3.48 (q, J = 7.1 Hz, 4H), 1.26 (t, J = 7.1 Hz, 6H). ESI-MS m / z: 246.1 [M+H] + .
Embodiment 3
[0059] Embodiment Three : Synthesis of compound 5a
[0060] Mix 3 ml of 2-pyrrolidone with 2.64 g of anthranilic acid, and slowly add 45 ml of phosphorus oxychloride dropwise under ice cooling. Then reflux for 7 h. After cooling, distill out phosphorus oxychloride, add ethyl acetate to dilute, adjust the solution to be weakly alkaline with sodium hydroxide solution, extract with ethyl acetate several times, collect the organic layer, dry with anhydrous magnesium sulfate, suction filter, and rotary evaporate , to get crude products. Purification by silica gel chromatography using petroleum ether / ethyl acetate (volume ratio 4 / 1) as the eluent afforded 1.51 g of white solid 5a in a yield of 42.1%: 1 H NMR (400 MHz, CDCl 3 )δ8.28 (d, J = 7.9 Hz, 1H), 7.77 - 7.69 (m, 1H), 7.65 (d, J = 8.1 Hz, 1H), 7.49 - 7.42 (m, 1H), 4.21 (t, J = 7.3 Hz, 2H), 3.19 (t, J = 7.9 Hz, 2H), 2.36 - 2.23 (m, 2H). ESI-MS m / z: 187.1 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com