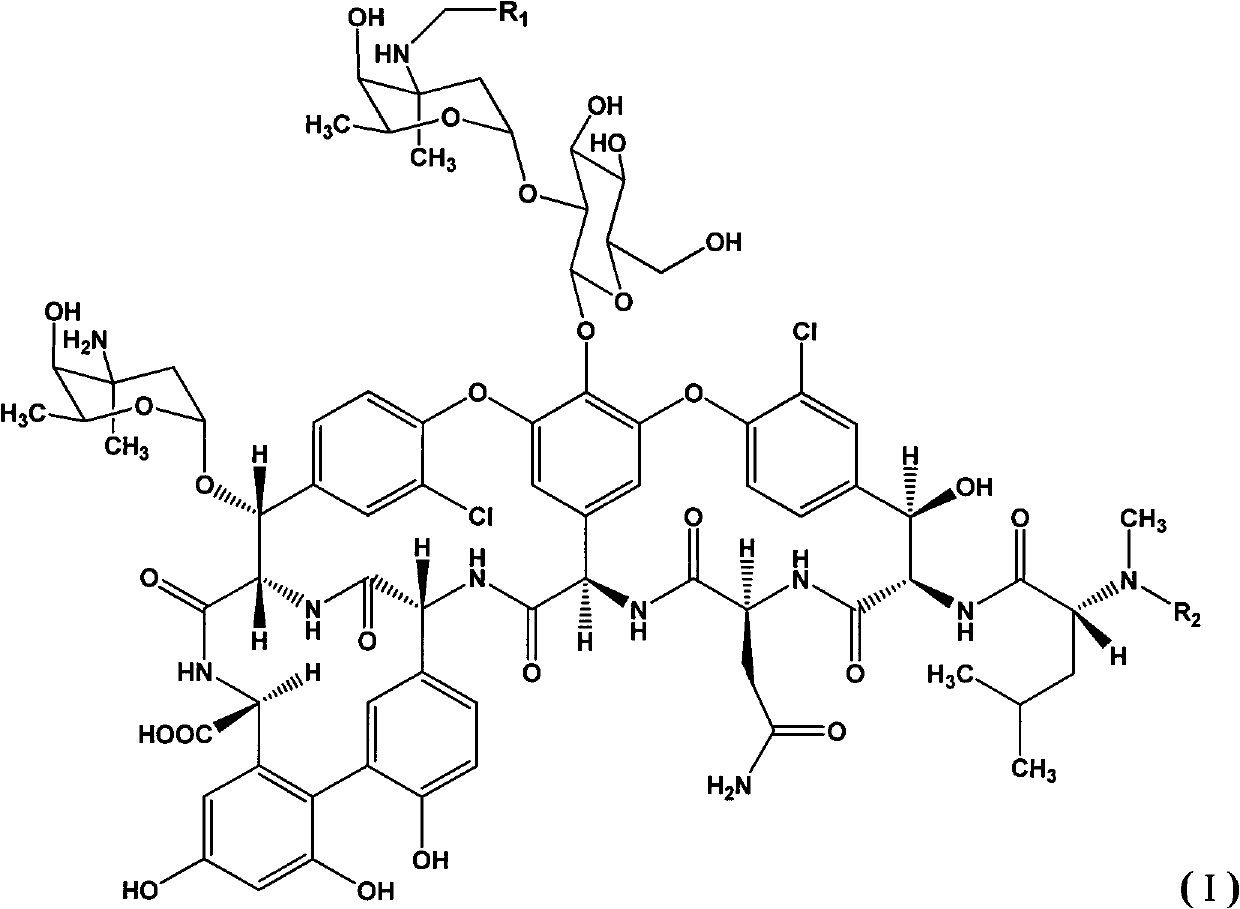
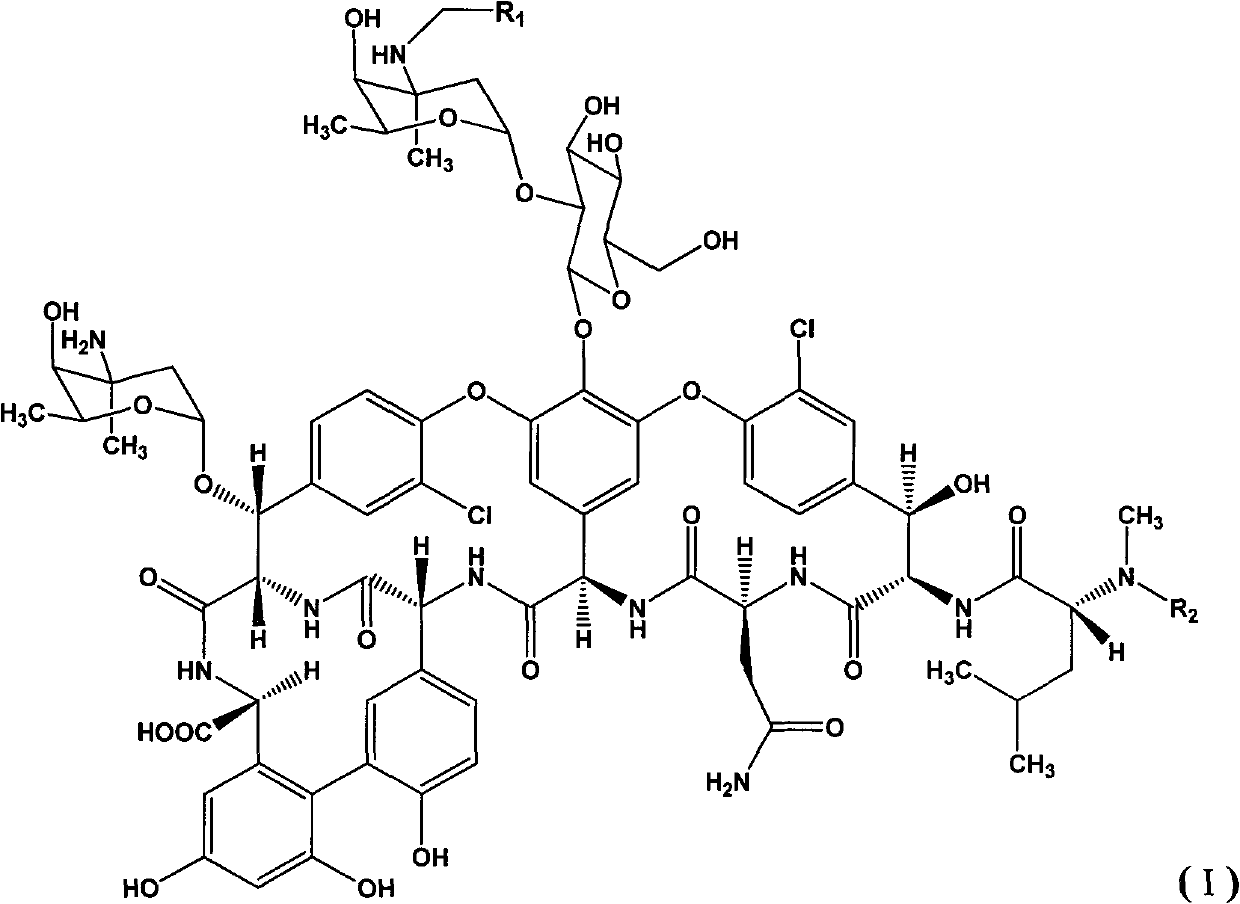
Novel glycopeptide antibiotic derivative and pharmaceutical composition, and preparation method and purpose thereof
A technology of glycopeptide antibiotics and derivatives, applied in the field of medicinal chemical synthesis, can solve problems such as the threat of clinical anti-infective treatment and the decrease of vancomycin sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the synthesis of compound 1
[0039] At room temperature, the compound represented by general formula (II) (500mg, 0.31mmol) was dissolved in 10mlDMF / methanol (1:1), and 4-bromobenzaldehyde (63mg, 0.34mmol) was added, and the reaction solution was heated at 60°C Heat and stir for 2 hours, cool to room temperature, add sodium cyanoborohydride (40mg, 0.62mmol), stir at room temperature for 2 hours, evaporate the methanol from the reaction solution under reduced pressure, pour the residue into 70ml acetone to precipitate precipitate, suction filter, wash with acetone , purified by preparative HPLC to obtain 100 mg of the final product (Compound 1), with a yield of 18.4%.
Embodiment 2
[0040] Embodiment 2: the synthesis of compound 1
[0041]The compound represented by general formula (II) (300mg, 0.19mmol) was dissolved in 6mlDMF / methanol (1:1), 4-bromobenzaldehyde (70mg, 0.38mmol) was added, the reaction solution was stirred at 0°C for 3 hours, and Sodium cyanoborohydride (24 mg, 0.38 mmol), stirred at room temperature for 2 hours, the reaction solution was evaporated under reduced pressure to remove methanol, the residue was poured into 50 ml of acetone to precipitate a precipitate, filtered by suction, washed with acetone, and purified by preparative HPLC to obtain the final product (Compound 1) 50 mg, yield 14.7%.
[0042] 1 H-NMR (400MHz, DMSO-d 6 +D 2 O)δ(ppm): 7.81(2H), 7.59-7.23(8H), 6.79(3H), 6.50(1H), 6.35(2H), 5.86-5.13(7H), 4.95-4.20(10H), 3.55- 2.50(6H), 2.45-2.00(4H), 1.90-0.95(15H), 0.89-0.84(6H).
Embodiment 3
[0043] Embodiment 3: the synthesis of compound 10
[0044] The compound represented by general formula (II) (500mg, 0.31mmol) was dissolved in 10ml of DMF / methanol (1:1), 4'-chloro-biphenylcarbaldehyde (100mg, 0.46mmol) was added, and the reaction solution was heated at 65°C After stirring for 2 hours, cool to room temperature, add sodium cyanoborohydride (40 mg, 0.62 mmol), and stir at room temperature for 2 hours. The reaction solution was evaporated to remove methanol under reduced pressure, and the residue was poured into 70 ml of acetone to precipitate a precipitate. Suction filtration, washing with acetone, Purified by preparative HPLC to obtain 130 mg of the final product (compound 10), with a yield of 23.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com