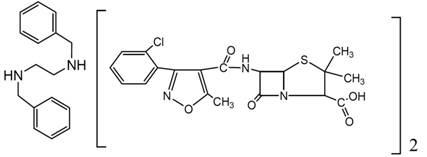
Preparation method of chlorazol benzathine benzylpenicillin
A technology for chlorpyrifosin and chlorfenacil is applied in the field of preparation of chlorfenacil, can solve the problems of complex production process, many reaction steps, low product yield and the like, and achieves reduction of toxicity, reduction of occlusion, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] At 25°C, add 10.0g of cloxacillin sodium into 100ml of purified water, stir to dissolve, control the pH between 6.0-8.0, add 100ml of 95vl% ethanol to make solution A;
[0026] Dissolve 4.5g of N,N'-dibenzylethylenediamine diacetic acid (DBED, the same below) in 45ml of purified water, add 45ml of 95vl% ethanol to make solution B.
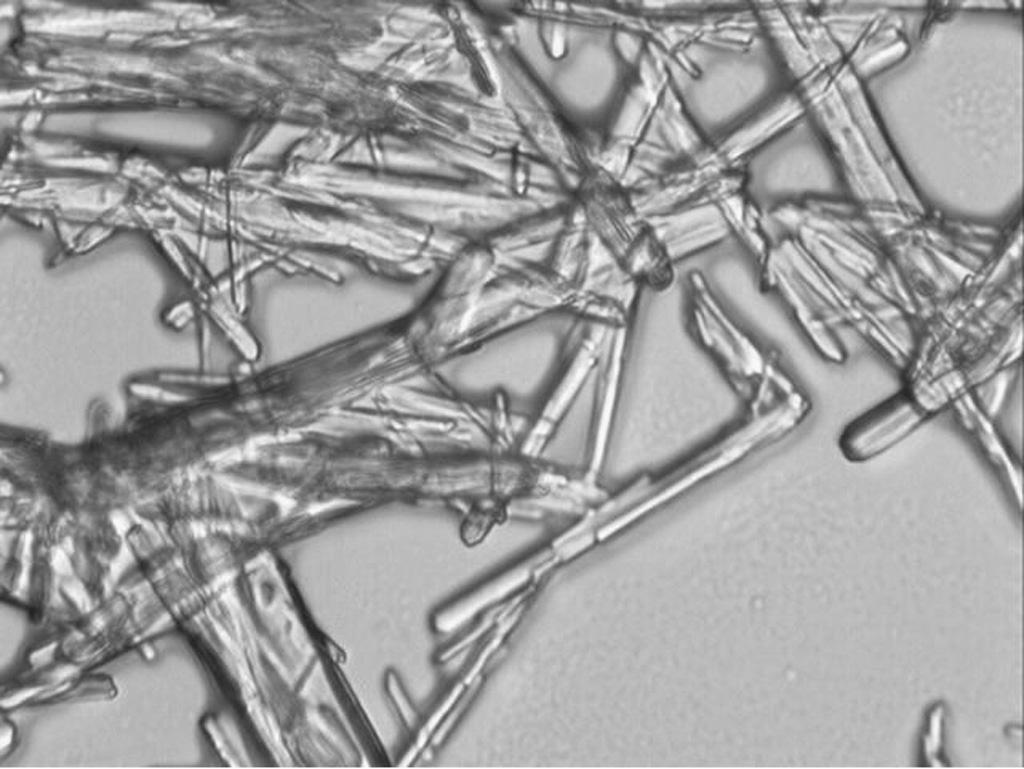
[0027] Pre-add 15% of the total amount of solution A to solution B, stir until the crystals appear, grow the crystals for 10 minutes, continue to feed solution A, complete the addition in 30 minutes, carry out the salt formation reaction for 4 hours, and obtain the cloxacin penicillin suspension , filtered, washed with ethyl acetate, suction filtered, and vacuum-dried at 50°C to obtain the finished product of benzathine penicillin with a yield of 90.0%, a grain size of 100 μm, a pH of 5.6, and a content of 95.1%.
Embodiment 2
[0029] At 25°C, add 15.0g of cloxacillin sodium into 30ml of purified water, stir to dissolve, control the pH between 6.0-8.0, add 10ml of 75vl% ethanol to make solution A;
[0030] Dissolve 6.4g of DBED in 40ml of purified water, add 20ml of 75% ethanol to make solution B.
[0031] Pre-add 30% of the total amount of solution A to solution B, stir until the crystals appear, grow the crystals for 10 minutes, continue to feed solution A, complete the addition in 30 minutes, carry out the salt formation reaction for 4 hours, and obtain chlorxazone penicillin suspension , filtered, washed with ethyl acetate, suction filtered, and vacuum-dried at 50°C to obtain the finished product of benzathine penicillin with a yield of 83.0%, a grain size of 100 μm, a pH of 5.6, and a content of 95.1%.
Embodiment 3
[0033] At 25°C, add 15.0g cloxacillin sodium into 150ml purified water, stir to dissolve, control the pH between 6.0-8.0, add 150ml 95% ethanol to make solution A;
[0034] Dissolve 6.4g of DBED in 12.8ml of purified water, add 6.7ml of 95% ethanol to make solution B.
[0035] Pre-add 15% of the total amount of solution B to solution A, stir until the crystals appear, grow the crystals for 30 minutes, continue to feed solution B, complete the addition in 30 minutes, carry out the salt formation reaction for 4 hours, and obtain cloxacin penicillin suspension , filtered, washed with ethyl acetate, suction filtered, and vacuum-dried at 50°C to obtain the finished product of benzathine penicillin with a yield of 90.0%, a crystal grain size of 100 μm, a pH of 5.6, and a content of 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com