Soluble polyfunctional (methyl) acrylate copolymer with alicyclic ring structure, curable resin composition and cured product
A curable resin, acrylate technology, applied in instruments, optics, optical components, etc., can solve the problems of reducing the strength and heat resistance of the resin composition, and achieve improved adhesion, low dispersion, and high light transmittance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Dimethylol tricyclodecane diacrylate (DMTCD) 1.6 moles (463.2ml), tricyclo[5.2.1.02,6]dec-8-yl methacrylate 1.2 moles (254.2ml), 1,4 -Butanediol diacrylate (BDDA) 1.2 moles (226.3ml), 2,4-diphenyl-4-methyl-1-pentene (αMSD) 0.4 moles (95.5ml), tert-dodecyl Put 2.4 moles (564.8ml) of mercaptan (TDM) and 600ml of toluene into a 3.0L reactor, and add 40mmol (11.5g) of tert-butyl peroxy-2-ethylhexanoate at 90°C to react 2 Hours and 45 minutes. After the polymerization reaction was stopped by cooling, the reaction mixture was poured into a large amount of hexane at room temperature to precipitate a copolymer. The obtained copolymer was washed with hexane, separated by filtration, dried, and weighed to obtain 536.4 g of copolymer A (yield: 73.2 wt %).
[0150] Mw of the obtained copolymer A was 34200, Mn was 5620, and Mw / Mn was 6.1. by carrying out 13 C-NMR, 1 H-NMR analysis and elemental analysis, copolymer A contains a total of 39.6 mol% of structural units (1) derived ...
Embodiment 2
[0160] 2.64 moles (764.3ml) of dimethyloltricyclodecane diacrylate, 0.24 moles (47.2ml) of tricyclo[5.2.1.02,6]dec-8-yl acrylate (DCPA), 1,4-butyl Diol diacrylate 0.96 moles (181.0ml), 2-hydroxypropyl acrylate (HOP-A) 0.96 moles (118.5ml), 2,4-diphenyl-4-methyl-1-pentene 0.48 moles ( 114.6ml), 3.12 moles of tert-dodecylmercaptan (734.3ml), and 720ml of toluene are dropped into a 3.0L reactor, and 62mmol (13.9g) of peroxy-2-ethylhexanoic acid tertiary butyl ester and allowed to react for 2 hours and 30 minutes. After the polymerization reaction was stopped by cooling, the reaction mixture was poured into a large amount of hexane at room temperature to precipitate a polymer. The obtained polymer was washed with hexane, separated by filtration, dried, and weighed to obtain 517.2 g of a copolymer B (yield: 71.5 wt %).
[0161] Mw of the obtained copolymer B was 39500, Mn was 7240, and Mw / Mn was 5.5. by carrying out 13 C-NMR, 1 H-NMR analysis and elemental analysis, copolymer ...
Embodiment 3~8 and comparative example 1~4
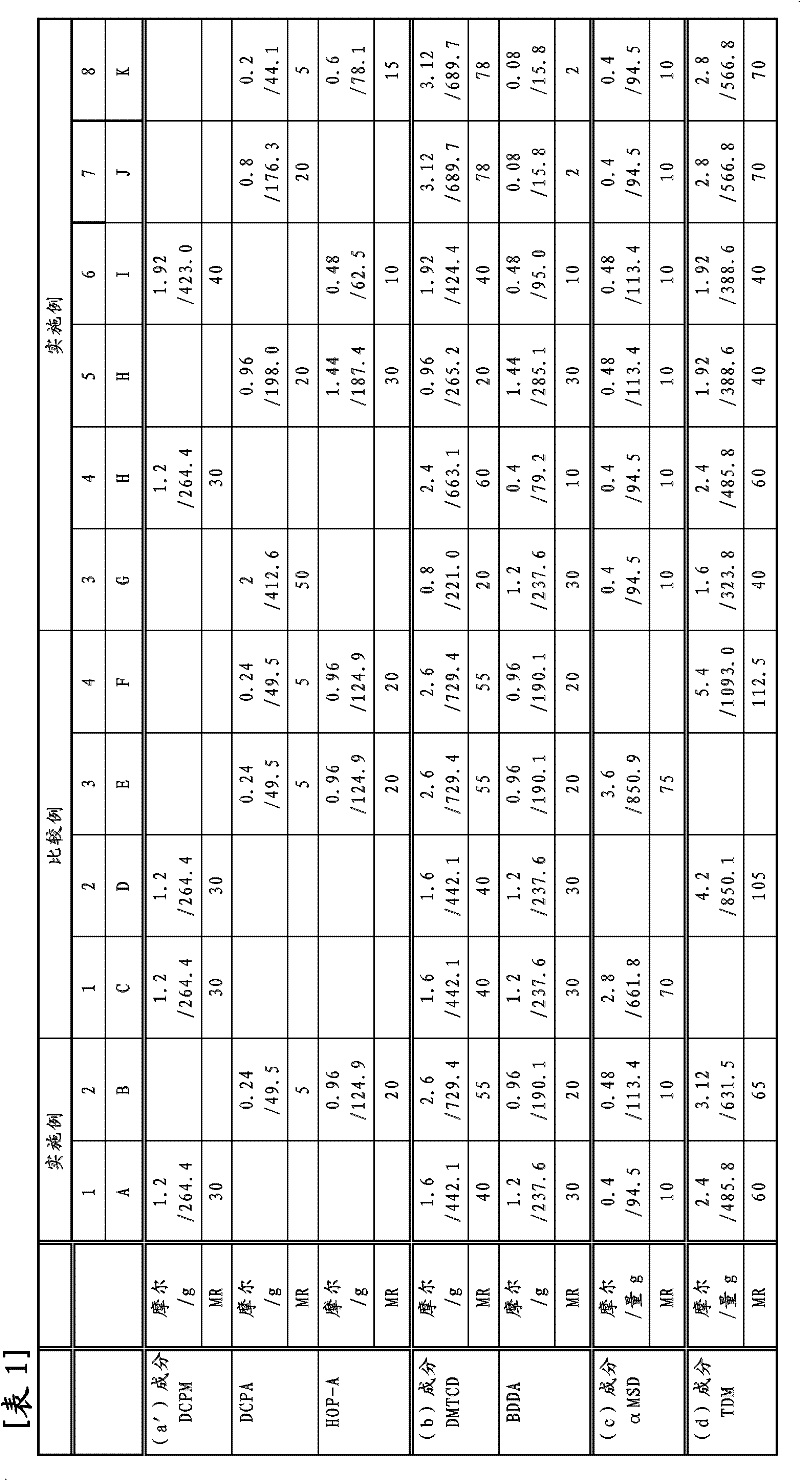
[0170] Using various monofunctional (meth)acrylates and bifunctional acrylates, polymerization was carried out in the same manner as in Example 1 with the raw material composition shown in Table 1.
[0171] Table 1 shows the amount of raw materials used in the reaction, and Table 2 shows the test results of the copolymer and its cured product. Unless otherwise specified, other reaction conditions and measurement conditions are the same as in Example 1. In Table 1, the amount of raw materials used is represented by mole and weight (g), and the form of record is mole / g. In addition, the mole fraction (MR) calculated the sum total of (a) component and (b) component as 100.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com